
EMERADE 500 micrograms Injectable Solution in Pre-filled Pen

How to use EMERADE 500 micrograms Injectable Solution in Pre-filled Pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Emerade 150 micrograms solution for injection in pre-filled pen EFG
Emerade 300 micrograms solution for injection in pre-filled pen EFG
Emerade 500 micrograms solution for injection in pre-filled pen
adrenaline
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Emerade and what is it used for
- What you need to know before you use Emerade
- How to use Emerade
- Possible side effects
- Storing Emerade
- Contents of the pack and other information
1. What is Emerade and what is it used for
Emerade is an auto-injector device that contains adrenaline in a solution for injection into a muscle (intramuscularly).
Adrenaline counteracts the fall in blood pressure in anaphylactic reactions. It also stimulates the heart and makes breathing easier.
Emerade is used as emergency treatment for severe allergic reactions (anaphylaxis) caused by food allergens, medicines, insect bites or stings, and other allergens, as well as those triggered by exercise or unknown causes.
2. What you need to know before you use Emerade
Your doctor will have explained when and how you should use Emerade. If you are not completely sure or have doubts, you should contact your doctor.
Warnings and precautions
Emerade can always be used during an allergic emergency. If you are allergic (hypersensitive) to sodium metabisulfite or any of the other components of Emerade, your doctor will explain in what circumstances you should use Emerade.
Consult your doctor before starting to use Emerade if you have:
- heart disease,
- high blood pressure,
- overactive thyroid gland,
- diabetes,
- a tumor of the adrenal gland,
- increased eye pressure (glaucoma),
- reduced kidney function,
- prostate disease,
- low potassium or high calcium levels in the blood.
If you have asthma, you may be at greater risk of a severe allergic reaction.
Anyone who has had an episode of anaphylaxis should see a doctor and have tests for substances they may be allergic to, in order to avoid them in the future. It is also important to note that an allergy to one substance can lead to allergies to other related substances.
If you are allergic to a food, it is essential that you check the composition of everything you eat (including medicines), as even small amounts can cause severe reactions.
There is also a greater risk of side effects in elderly people or pregnant women.
Instructions for use should be followed carefully to avoid accidental injection.
Emerade should be injected into the outer part of the thigh. It should not be injected into the buttock due to the risk of accidental injection into a vein.
Warnings
Accidental injection into the hands or feet can lead to reduced blood flow to the affected area. If an accidental injection occurs in these areas, you should go immediately to the nearest emergency department for treatment.
If you have a thick subcutaneous fat layer, there is a risk that a single dose of Emerade may not be sufficient. This could increase the need for a second injection of Emerade. Follow the instructions for use carefully as given in section 3.
Children
Emerade should not be used in children under 15 kg.
In the case of children under 15 kg, a dose below 150 micrograms cannot be administered with sufficient precision, and therefore its use is not recommended unless it is a potentially life-threatening situation and under medical advice.
Use in athletes:
This medicine contains adrenaline, which can produce a positive result in doping tests.
Using Emerade with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
This is particularly important if you are using any of the following:
- Antidepressants such as tricyclic antidepressants or monoamine oxidase inhibitors (MAOIs), as the effects of adrenaline may increase.
- Medicines for Parkinson's disease, such as catechol-O-methyltransferase inhibitors (COMT inhibitors), as they may increase the effect of adrenaline.
- Medicines that can cause the heart to beat irregularly (arrhythmias), such as digitalis and quinidine.
- Medicines called alpha or beta blockers for heart disease or nervous system disorders, as they may reduce the effect of adrenaline.
Diabetic patients should carefully monitor their blood glucose levels after using Emerade, as adrenaline can increase blood glucose levels.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Experience with the use of adrenaline during pregnancy is limited. However, even if you are pregnant, you should not avoid using Emerade in a life-threatening emergency situation.
You can breastfeed your baby after using Emerade.
Driving and using machines
It is unlikely that the ability to drive and use machines will be affected by the administration of an adrenaline injection, but it may be affected by a severe allergic reaction. If this ability is affected, you should not drive.
Emerade contains sodium metabisulfite
Rarely, sodium metabisulfite can cause severe hypersensitivity reactions or difficulty breathing (bronchospasm). If you are allergic (hypersensitive) to sodium metabisulfite, your doctor will explain in what circumstances you should use Emerade.
Emerade contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose, i.e., it is essentially "sodium-free".
3. How to use Emerade
Make sure you have received training in the use of Emerade and follow the instructions exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist.
Check the expiration date of your adrenaline auto-injectors and ask your doctor or nurse to prescribe new ones before they expire. Expired injectors may not work.
Emerade should be used immediately if signs or symptoms of a severe allergic reaction appear. Reactions can occur within minutes of contact with the allergen, and symptoms can include, for example, skin rash, flushing, or swelling. More severe reactions can also affect blood circulation and breathing.
Before the need to use Emerade arises, make sure you understand in what situations you should use it. If you are at risk of anaphylaxis, it is essential that you always carry two adrenaline pens with you at all times. Emerade should be stored in its original box, although during transport by the patient/caregiver, it is acceptable to store it in the specially designed container in which it is delivered. You should always carry the pen in this container to ensure protection of the pen and the label showing how to use it in an emergency situation. Always keep this leaflet in the case.
Dosage
The dose will be decided by your doctor, who will adjust the dose individually, for example, depending on your body weight.
Adults
Adults weighing less than 60 kg
The usual dose is 300 micrograms.
Adults weighing more than 60 kg
The usual dose is 300 to 500 micrograms.
Children and adolescents
Emerade 500 micrograms is not recommended for use in children.
Children between 15 and 30 kg in weight
The usual dose is 150 micrograms.
Children over 30 kg in weight
The usual dose is 300 micrograms.
Adolescents over 30 kg in weight
Follow the recommendations indicated for adults.
How to administer Emerade
Follow the instructions for use carefully to avoid accidental injection.
It is recommended that your family members, caregivers, or teachers also receive instructions on how to administer Emerade correctly.
Emerade should only be used by injecting it into the outer part of the thigh at the first signs of a severe allergic reaction. The injection is administered by pressing Emerade against the thigh. It can be administered through clothing. It should not be injected into the buttock.
If an Emerade pen does not activate, immediately make another attempt using greater force to press the pen against the intended injection site.
If you are not successful, proceed immediately to use your second pen.
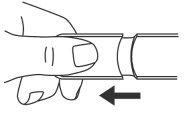
- Remove the cap.
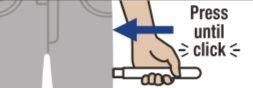 Place Emerade against the outer part of the thigh at a 90-degree angle and press firmly so that the needle protector retracts. You will hear a "click" when the device is activated and the needle penetrates the thigh.
Place Emerade against the outer part of the thigh at a 90-degree angle and press firmly so that the needle protector retracts. You will hear a "click" when the device is activated and the needle penetrates the thigh.
and
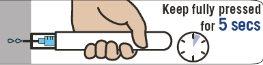 Hold Emerade firmly pressed against the thigh for 5 seconds. Then, gently massage the injection area.
Hold Emerade firmly pressed against the thigh for 5 seconds. Then, gently massage the injection area.
- Seek medical attention immediately.
The needle of Emerade is protected before, during, and after injection.
When the injection is complete, the needle protector of Emerade appears visibly longer, and the plunger is visible in the inspection window when the label is lifted.
Key messages for patients:
- Sometimes, a single dose of adrenaline is not enough to completely counteract the effects of a severe allergic reaction. For this reason, your doctor will prescribe two Emerade pens.
- If your symptoms have not improved or have worsened within 5-15 minutes after the first injection, either you or the person you are with should administer a second injection. For this reason, you should always carry two Emerade pens with you at all times.
- Emerade is designed as emergency treatment. Always seek medical attention immediately after using Emerade. Ask someone to stay with you until the ambulance arrives, in case you feel unwell again.
- Call 112, request an ambulance, and indicate that it is an 'anaphylaxis' case, even if symptoms seem to be improving. You will need to go to the hospital for observation or further treatment as needed. This is because the reaction may recur after some time. Bring the used pen with you.
- While waiting for the ambulance, you should lie down with your feet elevated, unless this position prevents you from breathing, in which case you should sit upright.
- Unconscious patients should be placed on their side in the recovery position.
After using the Emerade pen, following the instructions, the patient can verify if the pen has been activated. The images below (Fig.1-Fig.2) refer to all Emerade doses (150 micrograms, 300 micrograms, and 500 micrograms).
The unused Emerade pen (before activation) has the needle protector in its normal position (Fig. 1).
Fig 1.
The activated Emerade pen will have the needle protector extended (Fig. 2).

Fig. 2
If the needle protector is not extended, the pen has not been activated.
An Emerade pen that has been activated and has successfully administered a dose of adrenaline will show a colored plunger in the inspection window (which is revealed by lifting the label on the pen):
150 micrograms: yellow
300 micrograms: green
500 micrograms: blue.
If the inspection window still shows a clear liquid (adrenaline solution), the pen has not administered a dose of adrenaline correctly. The arrow on the pen label indicates where you can lift the label to reveal the inspection window.
Do not remove the cap unless you need to administer the injection.
After injection, some liquid may remain in the auto-injector. The auto-injector cannot be reused.
There are needle-free auto-injectors (demonstration pens) available for training.
Please consult your doctor.
If you use more Emerade than you should
If a higher dose is administered or if Emerade is accidentally injected into a blood vessel or hand, you should seek medical attention immediately.
Your blood pressure may rise sharply. Overdose can cause a sudden increase in blood pressure, irregular heartbeat, and fluid accumulation in the lungs, which can make breathing difficult.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 915620420, indicating the medicine and the amount ingested.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects are based on experience with the use of adrenaline, although their frequency cannot be estimated:
- heart problems, such as rapid and irregular heartbeat, chest pain,
- increased blood pressure, narrowing of blood vessels,
- sweating,
- nausea, vomiting,
- difficulty breathing,
- headache, dizziness,
- weakness, tremors,
- anxiety, hallucinations,
- fainting,
- changes in blood values, such as increased blood sugar, reduced potassium, and increased acidity
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use https://www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Emerade
Keep this medicine out of the sight and reach of children.
Store in the protective plastic container in which it is delivered. The plastic container containing the pen/pens can be stored in the outer box.
Store below 25°C. Do not freeze.
Do not use this medicine after the expiry date stated on the label and outer box. The expiry date is the last day of the month stated. After the expiry date, discard Emerade and replace it with a new pack. Periodically examine the solution through the inspection window by lifting the label to ensure that the solution remains transparent and colorless. Do not use this medicine if you notice that the solution has changed color or contains precipitates.
Check the auto-injector if it is dropped. Replace it if you notice any damage or leakage.
Medicines should not be disposed of via wastewater or household waste. Return unused medicines and their packaging to a pharmacy for proper disposal. By doing so, you will help protect the environment.
6. Container Content and Additional Information
Composition of Emerade
The active ingredient is adrenaline in the form of tartrate.
Emerade 150 micrograms releases 150 micrograms of adrenaline in 0.15 ml of solution.
Emerade 300 micrograms releases 300 micrograms of adrenaline in 0.3 ml of solution.
Emerade 500 micrograms releases 500 micrograms of adrenaline in 0.5 ml of solution.
The other components are: sodium chloride, sodium metabisulfite (E223), disodium edetate, hydrochloric acid, and water for injectable preparations.
Appearance of Emerade and Container Content
Emerade is an auto-injector that releases a single dose of adrenaline. Emerade contains a clear and colorless solution for injection, inside a glass syringe. Emerade does not contain latex.
The device is a white cylinder with a protector that covers the needle and a trigger mechanism.
Exposed needle length:
Emerade 150 micrograms: 16mm
Emerade 300 micrograms and 500 micrograms: 23mm.
Package sizes: 1 or 2 pre-loaded pens.
Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Holder
PharmaSwiss Ceská republika s.r.o.
Jankovcoca 1569/2c, 170 00 Prague 7.
Czech Republic.
Manufacturer
Rechon Life Science AB
Soldattorpsvägen 5, SE-216 13 Limhamn.
Sweden
Date of the Last Revision of this Leaflet: December 2021
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)http://www.aemps.gob.es/
You can access detailed and updated information about this medicinal product by scanning the QR code included in the leaflet with your mobile phone (smartphone). You can also access this information at the following internet address:
https://cima.aemps.es/info/80146
https://cima.aemps.es/info/80147
https://cima.aemps.es/info/80149

- Country of registration
- Average pharmacy price70.14 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EMERADE 500 micrograms Injectable Solution in Pre-filled PenDosage form: INJECTABLE, 1 mg/10 mlActive substance: epinephrineManufacturer: Laboratoire AguettantPrescription requiredDosage form: INJECTABLE, Epinephrine base 1 mg/mlActive substance: epinephrineManufacturer: B Braun Medical S.A.Prescription requiredDosage form: INJECTABLE, 1 mg/mlActive substance: epinephrineManufacturer: Laboratorios Basi Industria Farmaceutica S.A.Prescription required
Online doctors for EMERADE 500 micrograms Injectable Solution in Pre-filled Pen
Discuss questions about EMERADE 500 micrograms Injectable Solution in Pre-filled Pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











