
Adrenalina Aguettant

Ask a doctor about a prescription for Adrenalina Aguettant

How to use Adrenalina Aguettant
Leaflet accompanying the packaging: patient information
Adrenalina Aguettant, 1 mg/10 ml, solution for injection in a pre-filled syringe
Adrenalinum
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Adrenalina Aguettant and what is it used for
- 2. Important information before using Adrenalina Aguettant
- 3. How to use Adrenalina Aguettant
- 4. Possible side effects
- 5. How to store Adrenalina Aguettant
- 6. Contents of the pack and other information
1. What is Adrenalina Aguettant and what is it used for
Adrenalina Aguettant, solution for injection in a pre-filled syringe, contains the active substance adrenaline, which belongs to a group of medicines called adrenergic or dopaminergic agents.
The medicine is used:
- For the treatment of cardiac arrest (sudden cessation of heart function, breathing, and consciousness).
- For the treatment of acute anaphylactic reaction (severe shock or collapse caused by a severe allergic reaction).
2. Important information before using Adrenalina Aguettant
When not to use Adrenalina Aguettant
If you are allergic to the active substance or any of the other ingredients of this medicine (listed in section 6), when an alternative form of adrenaline or an alternative vasoconstrictor medicine is available.
Warnings and precautions
Adrenalina Aguettant is recommended for use in emergency situations. After administration of the medicine, constant medical supervision is necessary.
Precautions during use
The risk of side effects is increased if you have:
- a history of hyperthyroidism, even if it occurred in the past (thyroid gland disease),
- severe renal failure,
- hypercalcemia (elevated calcium levels in the blood),
- hypokalemia (low potassium levels in the blood),
- diabetes,
- heart disease or hypertension,
- brain damage or cerebral atherosclerosis,
- glaucoma (increased eye pressure),
- prostate diseases,
- you are elderly,
- you are pregnant.
Adrenalina Aguettant and other medicines
Tell your doctor about all medicines you are taking or have recently taken, as well as any medicines you plan to take. Medicines that may interact with Adrenalina Aguettant:
- halogenated anesthetic gases (gases used during anesthesia),
- certain antidepressants,
- medicines for hypertension, heart disease,
- medicines for diabetes.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor, pharmacist, or nurse before using this medicine.
Driving and using machines
Using Adrenalina Aguettant does not affect driving or operating machinery.
Adrenalina Aguettant contains sodium
The medicine contains 35.4 mg of sodium (the main component of common salt) in each pre-filled syringe. This corresponds to 1.77% of the maximum recommended daily intake of sodium in the diet for adults.
3. How to use Adrenalina Aguettant
Adrenalina Aguettant is administered by a doctor, nurse, or medical rescuer. They will decide what dose of the medicine is suitable for you and when and how it should be administered. In the case of life-threatening allergic reactions (acute anaphylactic reaction):
Recommended dose
Adults: the dose is 0.05 mg (0.5 ml of Adrenalina Aguettant 1:10,000 solution), repeated as needed until the desired response is achieved. In the case of cardiac arrest:
Adults:1 mg (10 ml of Adrenalina Aguettant 1:10,000 solution) administered intravenously or intracardially every 3-5 minutes until cardiac function is restored.
Children over 5 kg:10 micrograms/kg body weight (0.1 ml/kg body weight of Adrenalina Aguettant 1:10,000 solution) administered intravenously or intracardially every 3-5 minutes until cardiac function is restored. This medicine should not be used in doses less than 0.5 ml, so it should not be used in newborns and infants weighing less than 5 kg.
4. Possible side effects
Like all medicines, Adrenalina Aguettant can cause side effects, although not everybody gets them. The following side effects have been reported:
- anxiety,
- shortness of breath (difficulty breathing),
- nervousness,
- anxiety,
- sweating,
- palpitations (irregular or rapid heartbeat),
- tachycardia (rapid heart rate),
- pallor,
- seizures,
- weakness,
- dizziness,
- headache,
- nausea,
- vomiting,
- cold extremities,
- hallucinations,
- syncope,
- hyperglycemia (high blood sugar levels),
- hypokalemia (low potassium levels in the blood),
- metabolic acidosis (increased acidity of the blood),
- excessive pupil dilation.
Side effects in patients sensitive to adrenaline or after administration of adrenaline in high doses:
- cardiac arrhythmia (irregular heartbeat/cardiac arrest),
- hypertension (with the risk of cerebral hemorrhage),
- vasoconstriction (narrowing of blood vessels, e.g., skin, limbs, or kidneys),
- attacks of acute angina pectoris,
- risk of acute myocardial infarction.
Repeated local injections may cause tissue necrosis (tissue damage) at the injection site due to vasoconstriction.
In all cases, after administration of Adrenalina Aguettant, medical supervision is necessary.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, e-mail: [email protected]. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Adrenalina Aguettant
Keep the medicine out of sight and reach of children. Do not use this medicine after the expiry date stated on the label. The expiry date refers to the last day of the month stated. Your doctor or nurse will check this. Store in an aluminum sachet to protect from light and oxygen. Do not store above 25°C. Do not open the aluminum sachet until use. The product must be used immediately after opening the sachet. Do not freeze. Do not use sharp objects to open the sachet. Do not use this medicine if you notice that it has been partially used or has visible signs of damage. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Adrenalina Aguettant contains
The active substance is adrenaline hydrochloride: Each 1 ml of solution for injection contains 0.1 mg of adrenaline (as adrenaline hydrochloride). Each 10 ml pre-filled syringe contains 1 mg of adrenaline (as adrenaline hydrochloride). The other ingredients are sodium chloride, hydrochloric acid, sodium hydroxide, and water for injections.
What Adrenalina Aguettant looks like and contents of the pack
Adrenalina Aguettant is a clear, colorless solution in a 10 ml polypropylene pre-filled syringe, packaged individually in a transparent blister and placed in an aluminum sachet. Available packs: 1 or 10 pre-filled syringes, in a cardboard box. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Laboratoire Aguettant, 1 rue Alexander Fleming, 69007 LYON, France
Manufacturer:
Laboratoire Aguettant, 1 rue Alexander Fleming, 69007 LYON, France
Date of last revision of the leaflet: 03/2019
Detailed information on this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products.
Information intended for healthcare professionals only:
Adrenaline should be administered intravenously only by persons experienced in its use and in adjusting doses of vasoconstrictor substances, as part of standard clinical practice.
Cardiopulmonary resuscitation:
10 ml of 1:10,000 solution (1 mg) of adrenaline administered intravenously (IV) or intracardially (IO), repeated every 3-5 minutes until spontaneous circulation returns. Endotracheal administration should only be used as a last resort, if there is no access to any other route of administration, in a dose of 20-25 ml of 1:10,000 solution (2-2.5 mg). If cardiac arrest is preceded by a surgical procedure on the heart, adrenaline should be administered intravenously with great caution, in doses of 0.5 ml or 1 ml of 1:10,000 solution (50 micrograms or 100 micrograms), depending on the observed effect.
Acute anaphylactic reaction
Administer intravenously (bolus) 0.5 ml of 1:10,000 solution (0.05 mg), adjusting the dose according to the response to treatment. Adrenalina Aguettant 1 mg/10 ml (1:10,000), solution for injection in a pre-filled syringe, is not recommended for intramuscular administration in the treatment of acute anaphylactic reaction. For intramuscular administration, a 1 mg/ml (1:1000) solution should be used.
Pediatric population:
This medicine is not suitable for administration of a dose less than 0.5 ml, so it should not be used intravenously or intracardially in newborns and infants weighing less than 5 kg. Cardiac arrest in children:
Intravenously or intracardially (only in children over 5 kg): 0.1 ml/kg body weight of 1:10,000 solution (10 micrograms/kg body weight) of adrenaline, up to a maximum single dose of 10 ml of 1:10,000 solution (1 mg), repeated every 3-5 minutes until spontaneous circulation returns. Endotracheal administration should only be used as a last resort, regardless of the child's weight, if there is no access to any other route of administration, in a dose of 1 ml/kg body weight of 1:10,000 solution (100 micrograms/kg body weight) up to a maximum of 25 ml of 1:10,000 solution (2.5 mg).
It is essential to follow the instructions below:
The pre-filled syringe is for single use only, for one patient. The syringe should be discarded after use. Do not reuse.
Before administration, the product should be visually inspected for particulate matter and discoloration. Only a clear, colorless solution without particles or precipitates should be used. The product should not be used if the sachet or blister has been opened or if the tamper-evident protection on the syringe (plastic foil at the base of the protective cap) is damaged.
- 1) Open the aluminum sachet manually by tearing the notch. Do not use sharp objects to open the sachet.
- 2) Remove the pre-filled syringe from the sterile blister.
- 3) Press the plunger to release the stopper. The sterilization process may cause the stopper to stick to the syringe.
- 4) Twist the protective cap to break the tamper-evident protection. Do not touch the luer tip to avoid contamination.
- 5) Check that the protective cap has been completely removed. If not, replace the cap and twist again.
- 6) Remove any air by gently pressing the plunger.
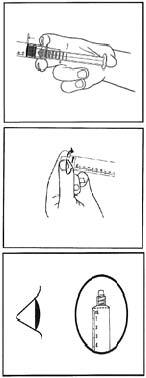
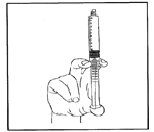
- 7) Connect the syringe to a device with access to a vein or connect it to a needle. Press the plunger to inject the required volume.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLaboratoire Aguettant
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Adrenalina AguettantDosage form: Solution, 1 mg/mlActive substance: epinephrinePrescription requiredDosage form: Solution, 300 mcg/0.3 mlActive substance: epinephrineManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription requiredDosage form: Solution, 0.15 mg/doseActive substance: epinephrineManufacturer: Meda Pharma GmbH & Co. KGPrescription required
Alternatives to Adrenalina Aguettant in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Adrenalina Aguettant in Ukraine
Alternative to Adrenalina Aguettant in Spain
Online doctors for Adrenalina Aguettant
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Adrenalina Aguettant – subject to medical assessment and local rules.










