
EDUNIX 32 mg PROLONGED-RELEASE TABLETS

How to use EDUNIX 32 mg PROLONGED-RELEASE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Edunix 32 mg prolonged-release tablets
Hydromorphone hydrochloride
For use in adults and adolescents from 12 years of age
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Edunix is and what it is used for
- What you need to know before you take Edunix
- How to take Edunix
- Possible side effects
- Storing Edunix
- Contents of the pack and other information
1. What Edunix is and what it is used for
Edunix is a strong pain reliever belonging to a group of medicines called opioid analgesics.
The tablets are used to treat severe pain.
2. What you need to know before you take Edunix
Do not take Edunix:
- if you are allergic to hydromorphone hydrochloride or any of the other ingredients of this medicine (listed in section 6);
- if you have breathing problems (respiratory depression or severe chronic obstructive pulmonary disease);
- if you have a severe and persistent airway disease with narrowing of the airways (e.g. severe asthma);
- in case of loss of consciousness (coma);
- if you have stomach problems or unexpected abdominal pain (acute abdomen);
- if you have an intestinal problem with lack of movement (paralytic ileus);
- if you are taking monoamine oxidase inhibitors (MAOIs - medicines for treating depression), or if you have taken this type of medicine in the last 2 weeks.
Warnings and precautions
Talk to your doctor or pharmacist before taking Edunix if any of the following apply to you:
- you or a family member have a history of abuse or dependence on alcohol, prescription drugs, or illicit substances ("addiction");
- you are a smoker;
- if you have ever had problems with your mood (depression, anxiety, or personality disorder) or have received psychiatric treatment for other mental illnesses;
- increased intracranial pressure or head injury;
- alcohol addiction or a severe reaction to stopping alcohol consumption (delirium tremens);
- a disease that causes seizures, such as epilepsy;
- a mental disorder called toxic psychosis;
- low blood pressure (hypotension) with low blood volume (hypovolemia);
- decreased consciousness, with a feeling of dizziness or fainting;
- biliary problems, biliary colic, or kidney problems (renal colic);
- pancreatitis (inflammation of the pancreas);
- intestinal problems, including inflammation, constriction, or obstruction of the intestine;
- enlarged prostate, causing difficulty urinating (prostatic hypertrophy);
- reduced function of the adrenal gland (e.g. Addison's disease). Your doctor will need to monitor your blood cortisol levels;
- underactive thyroid (hypothyroidism);
- breathing problems (such as chronic obstructive pulmonary disease or reduced respiratory capacity like asthma);
- elderly or debilitated patients;
- severe kidney or liver problems.
If you have or have had any of the above conditions or symptoms, talk to your doctor as you may need a lower dose of the medicine.
Abuse and dependence
Hydromorphone - like other strong pain relievers - has a potential for abuse. Repeated use of this medicine can lead to the development of psychological or physical dependence and abuse, which can lead to a potentially fatal overdose. It is essential that you inform your doctor if you think you may have developed dependence on this medicine. Therefore, patients with current or past history of alcohol or drug abuse should use Edunix with special caution.
Sudden interruption of therapy may cause a withdrawal syndrome. If treatment with hydromorphone is no longer needed, it may be advisable to gradually reduce the daily dose to avoid withdrawal symptoms.
Tolerance
This medicine contains hydromorphone, which is an opioid. Repeated use of opioid analgesics can reduce the effectiveness of the drug (your body gets used to the drug). This leads to the use of higher doses to achieve the desired pain relief. There may be cross-tolerance to other opioids; this means that when taking another opioid, you may also get used to that opioid.
Increased sensitivity to pain
Rarely, with the use of high doses, an increased sensitivity to pain (hyperalgesia) may occur, which will not respond to an additional increase in the dose of hydromorphone. In this case, your doctor will modify the treatment individually.
Respiratory disorders related to sleep
Edunix may cause respiratory disorders related to sleep, such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen levels in the blood). Symptoms may include interruptions in breathing during sleep, nighttime awakenings due to difficulty breathing, difficulty maintaining sleep, or excessive daytime sleepiness. If you or someone else observes these symptoms, talk to your doctor. Your doctor may consider reducing the dose.
Surgery
The use of Edunix is not recommended before or during the first 24 hours after surgery. After this time, Edunix should be used with caution, especially after abdominal surgery.
Paralysis of intestinal motility
Edunix should not be used in situations where paralysis of intestinal motility (paralytic ileus) may occur. If paralytic ileus is suspected or occurs during use, treatment with hydromorphone should be discontinued immediately.
Additional therapy for pain
If you are going to undergo additional therapy for pain (e.g. surgery, plexus block), you should not take hydromorphone during the 24 hours prior to surgery. From then on, the dose will be adjusted, which your doctor will take care of if necessary.
Switching to another opioid
You should be aware that once an effective dose of a particular opioid (a group of strong pain relievers to which Edunix belongs) has been applied, you should not be switched to another opioid without a medical evaluation and careful reassessment based on your needs. Otherwise, continuous pain relief will not be guaranteed.
Adrenal insufficiency
If your adrenal gland does not function properly (adrenal insufficiency), your doctor may want to monitor your plasma cortisol concentration and prescribe appropriate medications (corticosteroids).
Interference with normal hormone production
Edunix may interfere with the normal production of hormones produced by your body (such as cortisol or sex hormones). This can happen especially after taking high doses for long periods.
Note
The prolonged-release tablets should never be crushed and injected, as this could lead to serious side effects, with a fatal outcome.
Use in athletes
This medicine contains hydromorphone, which may produce a positive result in doping tests.
Children
This medicine is not recommended for use in children under 12 years of age. No clinical studies have been conducted on the use of hydromorphone in children.
Other medicines and Edunix
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Concomitant use of Edunix and sedative medicines such as benzodiazepines (which can help reduce anxiety and seizures, relax muscles, and induce sleep) increases the risk of drowsiness, breathing difficulties (respiratory depression), and coma, and can be potentially fatal. Therefore, concomitant use should only be considered when other treatment options are not possible. Concomitant use of opioids and drugs used to treat epilepsy, nerve pain, or anxiety (gabapentin and pregabalin) increases the risk of opioid overdose and respiratory depression, and can be potentially fatal.
However, if your doctor prescribes Edunix together with sedative medicines, your doctor will limit the dose and duration of concomitant treatment.
Tell your doctor about all sedative medicines you are taking and follow the recommended dose carefully. It may be useful to inform friends or family members who are aware of the signs and symptoms mentioned above. Contact your doctor when you experience these symptoms.
When taken with other medicines that depress brain function or alcohol, they can increase the side effects of hydromorphone or the other medicine, such as drowsiness or impaired respiratory function.
Such depressant medicines are:
- medicines for treating anxiety (e.g. tranquilizers);
- anesthetics that relax muscles (such as barbiturates);
- medicines for treating psychiatric or mental disorders (neuroleptics);
- medicines to help you sleep (such as hypnotics or sedatives);
- medicines for treating depression (antidepressants);
- medicines for treating allergies, dizziness, or nausea (antihistamines or antiemetics);
- other strong pain relievers (opioids). There may be cross-tolerance with other opioids. If you also take other strong pain relievers (opioids), you may develop tolerance to these pain relievers.
Do not take Edunix if you are taking medicines for treating depression called monoamine oxidase inhibitors (MAOIs) or during the 2 weeks after stopping these medicines (see section 2 "Do not take Edunix").
Concomitant use of hydromorphone with certain muscle relaxants (called muscle relaxants, which are usually injected or taken orally in the form of tablets) may lead to increased breathing difficulties (respiratory depression).
Taking Edunix with alcohol
Drinking alcohol while taking this medicine can make you feel more drowsy or increase the risk of serious side effects, such as shallow breathing with the risk of stopping breathing, and loss of consciousness. It is recommended not to drink alcohol while taking Edunix.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Edunix should not be used during pregnancy unless your doctor has specifically advised you to do so. There is limited or no data on the use of hydromorphone in pregnant women.
If Edunix is administered during pregnancy, it may affect uterine contractions. Additionally, there is a risk of breathing difficulties (respiratory depression) in the newborn.
Newborns may suffer from withdrawal syndrome (such as loud crying, tremors, convulsions, lack of appetite, and diarrhea) if their mothers have taken hydromorphone for a prolonged period during pregnancy.
Breastfeeding
Hydromorphone may pass into breast milk. Therefore, Edunix should not be used during breastfeeding. If its use is necessary, breastfeeding should be discontinued.
Driving and using machines
Hydromorphone has a moderate influence on the ability to drive and use machines. These effects are more noticeable at the start of treatment with hydromorphone, after increasing the dose, or changing medication. This is also likely if you combine Edunix with alcohol or other central nervous system depressants. If you are stabilized on a specific dose, you may not be affected necessarily. Therefore, you should consult your doctor to see if you are allowed to drive or use machinery.
Edunix contains sucrose
If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
Edunix contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per prolonged-release tablet; this is, essentially "sodium-free".
3. How to take Edunix
Follow exactly the administration instructions of this medication indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The dose depends on the severity of your pain and previous needs of analgesics.
Adults and adolescents over 12 years
Unless your doctor indicates otherwise, the initial dose in adults and adolescents over 12 years of age is 8 mg once a day (every 24 hours).
Important note: The daily dose of Edunix should not be administered more than once every 24 hours and should be taken approximately at the same time every day. The dose should not be increased in intervals of less than 2 days.
If adequate pain relief is not achieved, your doctor will increase the dose. In general, the minimum effective dose to relieve pain will be chosen for each individual case.
Edunix 32 mg prolonged-release tablets are not suitable for initial treatment with opioids; they should only be used in cases where lower doses do not provide sufficient pain relief.
If you think the action of this medication is too strong or too weak, inform your doctor.
Once an effective dose of Edunix has been adjusted, it should not be changed to other strong analgesics (opioid analgesic preparations). Clinical evaluation and careful dose adjustment by your doctor will be necessary. Otherwise, continuous pain relief is not guaranteed.
Elderly patients
Elderly patients may require a lower dose to achieve adequate analgesia.
Patients with hepatic or renal insufficiency
If you have renal or hepatic insufficiency, inform your doctor, as you may need lower doses to achieve pain control. Therefore, your hydromorphone dose should be adjusted with special care. This medication is not recommended for patients with severe hepatic insufficiency.
Use in children
The use of this medication is not recommended in children under 12 years of age. Clinical studies on the use of Edunix in children have not been conducted. Therefore, a recommended dose for this patient population cannot be provided. The dose will depend on the intensity of the pain and previous analgesic needs.
Method of administration: Oral.
Swallow the prolonged-release tablets with a sufficient amount of liquid (half a glass of water). The prolonged-release tablets can be divided into equal doses using the score lines. The prolonged-release tablets should not be chewed or crushed, as this can lead to rapid release of hydromorphone and symptoms of overdose (see "If you take more Edunix than you should").
How to open the child-resistant blister pack1. Cut a single dose along the perforation line of the blister pack.
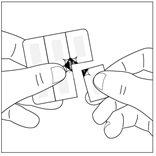
- In this way, the unscaled area is more accessible, where the perforation lines are marked.
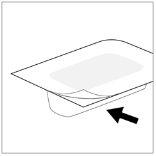
- Pull the unscaled part of the label to detach the aluminum cover.
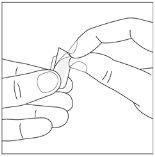
Duration of treatment
You should not take this medication for longer than necessary.
Your treatment should be regularly reevaluated with respect to pain relief and other effects, in order to achieve optimal pain treatment and consider the continuation of treatment.
If you take more Edunix than you should
If you have taken more prolonged-release tablets than prescribed, you should inform your doctor immediately. In case of overdose or accidental ingestion, consult your doctor or call the Toxicology Information Service; Telephone 91 562 04 20, indicating the medication and the amount taken.
The following symptoms may occur: constricted pupils (miosis), slow heartbeat (bradycardia), depressed breathing (respiratory depression), decreased blood pressure (hypotension), and increased drowsiness (somnolence) progressing to stiffness (stupor) or loss of consciousness (coma). Patients may develop pneumonia (possible symptoms: difficulty breathing, cough, and fever) caused by inhalation of vomit or solids.
In severe cases of circulatory collapse or deep unconsciousness (coma), it can cause death. Under no circumstances should you expose yourself to situations that require high concentration, such as driving a car.
In case of overdose, the following measure may be advisable until the doctor arrives: stay awake, give breathing instructions, provide respiratory assistance.
If you forget to take Edunix
Do not take a double dose to make up for forgotten doses.
If you take a lower dose than indicated or if you forget to take the prolonged-release tablet, consequently, pain relief will be insufficient or will cease completely. If you forget to take a tablet, you can take the dose immediately and start a new 24-hour schedule. In principle, you should not take more than 1 Edunix prolonged-release tablet once every 24 hours.
If you interrupt treatment with Edunix
Do not stop taking this medication without consulting your doctor. If you stop taking Edunix after prolonged use, you may experience withdrawal symptoms (e.g., agitation, anxiety, nervousness, difficulty sleeping, involuntary movements, tremors, and gastrointestinal upset). If therapy is no longer necessary, treatment should be gradually discontinued by reducing the dose.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Side effects or signs to consider and measures to take when these side effects or signs occur:
- Sudden severe allergic reactions that can be life-threatening:
- Hypersensitivity reactions (frequency cannot be estimated from available data);
- Anaphylactic reactions (frequency cannot be estimated from available data).
Contact your doctor immediately if you notice any of the following symptoms: difficulty breathing, swelling of eyelids, face, lips, mouth, or throat, skin rash or itching, especially those that cover the entire body.
If you have a severe allergic reaction, stop using Edunix prolonged-release tablets and see a doctor immediately. You may need urgent medical treatment.
Slow and weak breathing (respiratory depression) is the most serious side effect of opioid overdose.
Most patients become constipated if they take hydromorphone. If you have constipation or nausea (feeling of discomfort), your doctor will take appropriate measures. You can counteract the side effect of constipation by using preventive measures (e.g., increased fluid intake, fiber-enriched foods such as fruit, vegetables, and whole grains). If you already had constipation problems before starting to take your prolonged-release tablets, you should take laxatives from the beginning. Please consult your doctor.
If you experience dizziness or vomiting (which occurs frequently, especially at the start of treatment), your doctor may prescribe the appropriate medication.
Other possible side effects:
Very common (may affect more than 1 in 10 people):
- Constipation, nausea.
- Dizziness, drowsiness.
Common (may affect up to 1 in 10 people):
- Discomfort.
- Decreased appetite, loss of appetite.
- Anxiety, confusion, insomnia.
- Headache.
- Dry mouth, abdominal pain or discomfort.
- Itching, sweating.
- Sudden need to urinate.
- Feeling of weakness.
Uncommon (may affect up to 1 in 100 people):
- Withdrawal symptoms such as agitation, anxiety, nervousness, difficulty sleeping, involuntary movements (e.g., tremors), or gastrointestinal upset (when stopping Edunix).
- Indigestion, diarrhea, taste disorders.
- Hyperexcitability, depression, mood changes (euphoria), hallucinations, nightmares.
- Tremors, muscle spasms, abnormal skin sensations (pinching and needles).
- Visual disturbances.
- Low blood pressure.
- Breathing difficulties.
- Changes in blood tests that show how your liver is working (increased liver enzymes).
- Skin rash, hives.
- Difficulty urinating.
- Decreased sexual desire, impotence.
- Fatigue, generally feeling unwell, swelling of hands, ankles, or feet (fluid accumulation in tissue).
Rare (may affect up to 1 in 1,000 people):
- Drowsiness up to sedation, lack of energy.
- Increased heart rate.
- Slow heart rate, abnormal heart rhythm.
- Respiratory muscle convulsions, weakening and slowing of breathing (respiratory depression), breathing difficulties, and wheezing (bronchospasm).
- Changes in blood tests that show how your pancreas is working.
- Flushing of the face.
Frequency not known (cannot be estimated from available data):
- Exhaustion, uncontrolled muscle movements, increased sensitivity to pain (hyperalgesia, see section 2 "Warnings and precautions").
- Loss of intestinal movement (paralytic ileus).
- Biliary colic.
- Drug addiction, mood changes (dysphoria), restlessness.
- Pupil constriction (miosis).
- Feeling of heat.
- Itchy skin rash (hives).
- Need to take higher doses (called habituation or drug tolerance).
- Sleep apnea (breathing interruptions during sleep).
Drug withdrawal syndrome in newborns whose mothers have taken Edunix during pregnancy (see section "Pregnancy and lactation")
If you experience any side effect, inform your doctor or pharmacist. This includes side effects not mentioned in this leaflet.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the notification system included in the Spanish Pharmacovigilance System for Human Use Medicines. Website: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Edunix
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the packaging and blister pack, after CAD. The expiration date is the last day of the month indicated.
This medication does not require special storage conditions.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the SIGRE collection point in your pharmacy. Ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Edunix
- The active ingredient is hydromorphone hydrochloride.
Each prolonged-release tablet contains 32 mg of hydromorphone hydrochloride (equivalent to 28.38 mg of hydromorphone).
- Other components are:
Core of the tablet: Sugar spheres, hypromellose, ethylcellulose, hypromellose, triethyl citrate, talc, sodium carmellose, microcrystalline cellulose, magnesium stearate, anhydrous colloidal silica.
Coating of the tablet: Polyvinyl alcohol, macrogol 4000, talc, iron oxide (III) (E172)
Appearance of the product and package contents
Edunix 32 mg prolonged-release tablets are dark red, oblong, biconvex, 18 x 8.5 mm in size, with a score line on both sides.
The tablets can be divided into equal doses.
They are presented in child-resistant aluminum/PVC-PE-PVDC blister packs.
Package sizes: 10, 14, 20, 28, 30, 50, 56, 60, 98, 100 prolonged-release tablets.
Not all package sizes may be marketed.
Marketing authorization holder
Aristo Pharma GmbH
Wallenroder Str. 8-10
13435 Berlin
Germany
Manufacturer
Aristo Pharma GmbH
Wallenroder Str. 8-10
13435 Berlin
Germany
Or
Laboratorios Medicamentos Internationales, S.A.
C/Solana, 26
Torrejón de Ardoz
28850 Madrid
Spain
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Aristo Pharma Iberia, S.L.
C/ Solana, 26
28850, Torrejón de Ardoz
Madrid, Spain
This medication is authorized in the Member States of the European Economic Area under the following names:
Germany: Hydromorphon Aristo long 32 mg Retardtabletten
Spain: Edunix 32 mg prolonged-release tablets
Date of the last revision of this leaflet: December 2022
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price151.32 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EDUNIX 32 mg PROLONGED-RELEASE TABLETSDosage form: MODIFIED-RELEASE TABLET, 16 mgActive substance: hydromorphoneManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 2.6 mg/mlActive substance: hydromorphoneManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: MODIFIED-RELEASE TABLET, 4 mgActive substance: hydromorphoneManufacturer: Aristo Pharma GmbhPrescription required
Online doctors for EDUNIX 32 mg PROLONGED-RELEASE TABLETS
Discuss questions about EDUNIX 32 mg PROLONGED-RELEASE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












