
EDUNIX 2.6 mg/ml ORAL SOLUTION

How to use EDUNIX 2.6 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Edunix 2.6 mg/ml Oral Solution
Hydromorphone Hydrochloride
For use in adolescents from 12 years and adults
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Edunix and what is it used for
- What you need to know before you take Edunix
- How to take Edunix
- Possible side effects
- Storage of Edunix
- Contents of the pack and other information
1. What is Edunix and what is it used for
The oral solution has been prescribed to you for the relief of intense pain.
It contains the active ingredient hydromorphone, which is a strong analgesic ('pain reliever') belonging to a group of medicines called opioid analgesics.
2. What you need to know before you take Edunix
Do not take Edunix
- if you are allergic to hydromorphone hydrochloride or any of the other ingredients of this medicine (listed in section 6, Further information);
- if you have severe respiratory problems, such as severe chronic obstructive pulmonary disease, respiratory depression, or severe asthma. Symptoms may include shortness of breath, coughing, or slower and weaker breathing than expected;
- if you have stomach problems or unexpected abdominal pain (acute abdomen);
- if you have a bowel problem with lack of movement (paralytic ileus);
- if you are taking monoamine oxidase inhibitors (e.g., tranylcypromine, phenelzine, isocarboxazid, moclobemide, and linezolid), or if you have taken this type of medicine in the last 2 weeks.
Warnings and Precautions
Consult your doctor or pharmacist before starting to take hydromorphone hydrochloride if any of the following apply to you:
- breathing problems (such as chronic obstructive pulmonary disease or reduced respiratory capacity, e.g., asthma); symptoms may include shortness of breath and coughing; having difficulty sleeping (sleep apnea);
- you have a severe headache or feel unwell due to increased pressure in the brain or a head injury (e.g., due to a brain disease). This is because the oral solution may worsen symptoms or hide the extent of a head injury;
- you suffer from seizures, convulsions, or epileptic fits;
- you have a mental disorder due to poisoning (toxic psychosis);
- you have low blood pressure (hypotension);
- you feel weak or dizzy;
- you have biliary problems, biliary colic, or kidney problems;
- you have abdominal pain or discomfort of a colic type;
- you have pancreatitis (which can cause severe abdominal and back pain);
- you have intestinal obstruction or inflammatory bowel disorders;
- you have an enlarged prostate, which causes difficulty urinating (in men);
- you have reduced function of the adrenal gland, e.g., Addison's disease (the adrenal gland does not work properly);
- you have low thyroid activity (hypothyroidism);
- you have severe kidney or liver problems;
- if you or a family member have a history of abuse or dependence on alcohol, prescription medicines, or illicit substances ("addiction");
- if you smoke;
- if you have ever had problems with your mood (depression, anxiety, or personality disorder) or have received psychiatric treatment for other mental illnesses;
- you have withdrawal symptoms such as agitation, anxiety, tremors, or sweating when stopping alcohol or drugs;
- elderly or debilitated patients;
- you suffer from constipation.
Respiratory disorders related to sleep
Hydromorphone may cause respiratory disorders related to sleep, such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen levels in the blood). Symptoms may include interruptions in breathing during sleep, nighttime awakenings due to difficulty breathing, difficulty maintaining sleep, or excessive sleepiness during the day. If you or someone else observes these symptoms, consult your doctor. Your doctor may consider reducing the dose.
You may experience hormonal changes while taking this oral solution. Your doctor may want to monitor these changes.
You may experience increased sensitivity to pain despite taking high doses of the oral solution (hyperalgesia). Your doctor will decide if you need a dose change or a change in strong pain reliever ('painkiller').
If you are going to have surgery, inform your doctor that you are taking this oral solution.
The contents of the oral solution should never be injected, as it may cause serious side effects that can be fatal.
This medicine contains hydromorphone, which is an opioid. Repeated use of opioid analgesics may reduce the effectiveness of the medicine (your body gets used to the medicine).
Repeated use of hydromorphone may cause dependence and abuse, which can lead to a potentially fatal overdose. It is essential that you inform your doctor if you think you may have developed dependence on this medicine.
Other medicines and Edunix
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
If you take this oral solution with another medicine, the effect of the oral solution or the other medicine may be altered.
This oral solution should not be used with a monoamine oxidase inhibitor or if you have taken this type of medicine in the last two weeks (see section 2 'Do not take Edunix').
Tell your doctor or pharmacist if you are taking:
- medicines that help you sleep or calm down (e.g., tranquilizers, hypnotics, or sedatives, including benzodiazepines);
- medicines known as barbiturates to treat seizures or to help you sleep;
- medicines to prevent or relieve nausea and vomiting;
- medicines to prevent or relieve allergy symptoms (antihistamines);
- medicines to treat depression;
- medicines to treat psychiatric or mental disorders (antipsychotics, such as phenothiazines);
- other strong pain relievers ('painkillers').
Tell your doctor if you have recently been given an anesthetic.
Taking hydromorphone and benzodiazepines (which can help reduce anxiety and seizures, relax muscles, and induce sleep) at the same time increases the risk of drowsiness, breathing difficulties (respiratory depression), and coma, and can be potentially fatal. Therefore, concomitant use should only be considered when other treatment options are not possible. Concomitant use of opioids and medicines used to treat epilepsy, nerve pain, or anxiety (gabapentin and pregabalin) increases the risk of opioid overdose and respiratory depression, and can be potentially fatal.
However, if your doctor prescribes Edunix with sedative medicines, your doctor must limit the dose and duration of concomitant treatment.
Tell your doctor about all sedative medicines you are taking and follow your doctor's recommended dose carefully. It may be helpful to inform friends or family members who are aware of the signs and symptoms indicated above. Contact your doctor when you experience these symptoms.
Taking Edunix with food, drinks, or alcohol
This oral solution can be taken with or without food, but it is recommended not to drink alcohol while taking Edunix.
Drinking alcohol during treatment with this oral solution may cause drowsiness or negatively affect your breathing.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Edunix should not be used during pregnancy and childbirth unless your doctor has specifically indicated it. Depending on the dose and duration of treatment with hydromorphone, it may cause slow and shallow breathing (respiratory depression) or withdrawal symptoms in the newborn. Newborns may suffer from withdrawal symptoms (such as loud crying, nervousness, seizures, lack of appetite, and diarrhea) if their mothers have taken hydromorphone for a prolonged period during pregnancy.
Breast-feeding
Hydromorphone may pass into breast milk. Therefore, Edunix should not be used during breast-feeding.
Driving and using machines
This oral solution may cause a number of side effects, such as drowsiness, which may affect your ability to drive or use machines (see section 4 "Complete list of side effects"). These effects are more pronounced when starting to take the oral solution or when increasing the dose. If you are affected, do not drive or use machines.
Edunix contains methyl parahydroxybenzoate
It may cause allergic reactions (possibly delayed) because it contains methyl parahydroxybenzoate.
Edunix contains sucrose
This medicine contains sucrose. If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
It may cause tooth decay.
Edunix contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per prolonged-release tablet; this is essentially "sodium-free".
3. How to take Edunix
Always take the oral solution exactly as your doctor has told you. The label on your medicine will tell you how much oral solution to take and how often.
Do not exceed the dose recommended by your doctor. If in doubt, consult your doctor or pharmacist again.
Adults and adolescents over 12 years
The usual starting dose is 0.5 ml (1.3 mg) or 1.0 ml (2.6 mg) of oral solution every 4 hours. However, your doctor will prescribe the dose needed to relieve your pain. If you notice that you are still in pain while taking this oral solution, discuss this with your doctor.
Children under 12 years
This medicine is not recommended for use in children under 12 years.
Elderly patients and patients with kidney or liver problems
Tell your doctor if you have kidney or liver problems. Your doctor may prescribe a lower dose of this oral solution if you are an elderly patient or have kidney or liver problems.
Method of administration
This medicine is for oral use.
- Open the bottle: Press the child-resistant cap and turn it counterclockwise (Fig. 1). Note: Keep the cap handy to close the bottle immediately after each use.
- Only for the first use:Place the bottle on a flat surface. Firmly push the plastic adapter into the neck of the bottle with your thumb, if supplied separately (fig. 2). If the adapter is already attached to the bottle, skip the step in Fig. 2.
- Take the syringe and check that the syringe plunger is fully pressed.
- Hold the bottle upright and firmly insert the applicator syringe into the plastic adapter (Fig. 3).
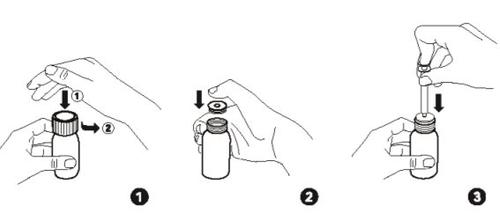
- Turn the bottle and syringe upside down.
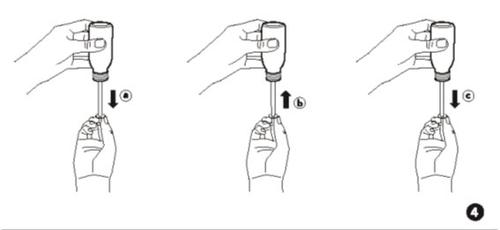
- Slowly pull the plunger down to the stop, to fill the syringe with the medicine (Fig. 4a). Then, push the plunger completely up to remove any air bubbles that may be inside the applicator syringe (Fig. 4b).
- Then, slowly pull the plunger back to the amount (mark) you need for your dose (Fig. 4c).
- Turn the whole bottle with the syringe back upright. With your thumb holding the bottle, put the adapter back and remove the syringe from the bottle (Fig. 5).
- Now, you can take the dose of the medicine directly from the syringe. To do this, remember to sit upright and slowly press the syringe plunger to swallow the dose correctly. You can also mix the dose of the medicine in a non-alcoholic drink. If you do, you must take this drink immediately after mixing.
- Close the bottle after use with the child-resistant cap. Note: The adapter remains in the bottle (Fig. 6).
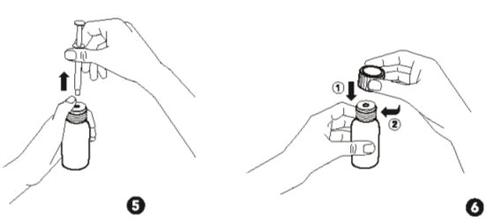
- Cleaning:After use, clean the outside of the syringe with a clean, dry cloth.
If you take more Edunix than you should or if someone accidentally takes your oral solution
If you have taken more oral solution than you should, call your doctor or hospital immediately. In case of overdose or accidental ingestion, consult your doctor or call the Toxicology Information Service; Telephone 91 562 04 20, indicating the medicine and the amount taken.
People who have taken an overdose may feel very drowsy, sick, or dizzy and may also develop constricted pupils, breathing problems, low blood pressure, or pneumonia caused by inhaling vomit or foreign objects (symptoms may include shortness of breath, coughing, and fever). In severe cases of circulatory collapse or deep unconsciousness (coma), it can cause death. When you go to the doctor, make sure to bring this leaflet and any remaining oral solution to show the doctor.
If you have taken too much oral solution, do not expose yourself to situations that require high concentration, such as driving a car.
If you forget to take Edunix
If you forget to take a dose, take it as soon as you remember and continue as before. Do not take two doses within a 4-hour period. Do not take a double dose to make up for the forgotten dose.
If you stop taking Edunix
Do not stop taking this oral solution suddenly unless your doctor tells you to. If you want to stop taking your oral solution, discuss it with your doctor first. They will tell you how to do it, usually by gradually reducing the dose so that you do not experience side effects. Withdrawal symptoms such as agitation, anxiety, nervousness, difficulty sleeping, unusual hyperactivity, tremors, or gastrointestinal disorders (e.g., stomach upset) may occur if you stop taking this oral solution suddenly.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
This medicine may cause allergic reactions (hypersensitivity) that can be severe (anaphylactic reactions). The frequency of these reactions is unknown. Inform your doctor immediately if you have difficulty breathing, swelling of the eyelids, face, lips, mouth, or throat, or any skin rash or itching, especially those that cover your entire body.
Slow and weak breathing (respiratory depression) is the most serious adverse effect of opioid overdose.
As with all potent analgesics, there is a risk of becoming addicted or dependent on this oral solution.
Very Common(may affect more than 1 in 10 people):
- Constipation (your doctor may prescribe a laxative for this problem).
- Feeling of discomfort.
- Dizziness, drowsiness.
Common(may affect up to 1 in 10 people):
- Discomfort (usually disappears after a few days, but your doctor may prescribe medication if the problem persists).
- Anxiety, confusion.
- Difficulty sleeping.
- Dry mouth, loss of appetite, abdominal pain, or abdominal discomfort.
- Headache.
- Feeling of weakness.
- Itching of the skin.
- Sweating.
- Sudden need to urinate.
Uncommon(may affect up to 1 in 100 people):
- Withdrawal symptoms (see section 3 "If you stop treatment with Edunix").
- Indigestion, diarrhea, taste disorders.
- Depression, feeling of extreme happiness (euphoria), hallucinations, nightmares.
- Blurred vision.
- Agitation.
- Tremors, muscle spasms, tingling, or numbness.
- Low blood pressure.
- Difficulty breathing.
- Decreased sexual desire, impotence.
- Skin rash, hives.
- Swelling of hands, ankles, or feet (fluid accumulation in tissue).
- Difficulty urinating.
- General discomfort.
- Fatigue.
- Worsening of liver function tests (in a blood test).
Rare(may affect up to 1 in 1,000 people):
- Aggression.
- Feeling more drowsy than usual.
- Lack of energy.
- Fast heart rate, slow heart rate, palpitations.
- Wheezing or difficulty breathing.
- Changes in pancreatic function (in a blood test).
Frequency Not Known(cannot be estimated from available data):
- Sleep apnea (interruptions of breathing during sleep).
- Seizures, convulsions.
- Mood changes (dysphoria), restlessness.
- Increased sensitivity to pain.
- Intestinal failure (paralytic ileus).
- Pupil constriction.
- Feeling of heat.
- Uncontrolled muscle movements.
- Itchy skin rash (hives).
- Need to take higher doses to achieve the same level of pain relief (tolerance).
- Drug withdrawal syndrome in newborns whose mothers have taken Edunix during pregnancy (see section 2).
If you experience any adverse effect, inform your doctor or pharmacist. This includes adverse effects not mentioned in this prospectus.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the notification system included in the Spanish Pharmacovigilance System for Human Use Medicines. Website: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Edunix
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging and label, after CAD. The expiration date is the last day of the month indicated.
Store below 25°C. Keep in the original packaging.
Do not use after 2 months after the first opening of the bottle.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Edunix
- The active ingredient is hydrochloride of hydromorphone. Each ml contains 2.6 mg of hydrochloride of hydromorphone equivalent to 2.32 mg of hydromorphone.
- The other components are: methyl parahydroxybenzoate (E-218), sucrose, glycerol 99% (E-422), citric acid monohydrate (E-330), sodium citrate (E-331), and purified water.
Appearance of the Product and Package Contents
Transparent, colorless, slightly viscous solution, with a sweet taste and smell. It includes a dosing syringe for dosing.
The oral solution is packaged in brown glass bottles of 20, 50, or 100 ml, closed with a child-resistant screw cap with a tamper-evident ring. The bottle is inserted into a folding box along with a 3 ml plastic dosing syringe (graduation of 0.1 ml).
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Aristo Pharma GmbH
Wallenroder Str. 8-10
13435 Berlin
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Aristo Pharma Iberia, S.L.
C/ Solana, 26
28850, Torrejón de Ardoz
Madrid, Spain
This medicine is authorized in the member states of the European Economic Area under the following names:
Germany: Hydromorphon Aristo 2,6 mg/ml Lösung zum Einnehmen
Spain: Edunix 2,6 mg/ml oral solution
Malta: Hydromorphone Aristo 2,6 mg/ml oral solution
Date of the Last Revision of this Prospectus: January 2025
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EDUNIX 2.6 mg/ml ORAL SOLUTIONDosage form: MODIFIED-RELEASE TABLET, 16 mgActive substance: hydromorphoneManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: MODIFIED-RELEASE TABLET, 32 mgActive substance: hydromorphoneManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: MODIFIED-RELEASE TABLET, 4 mgActive substance: hydromorphoneManufacturer: Aristo Pharma GmbhPrescription required
Online doctors for EDUNIX 2.6 mg/ml ORAL SOLUTION
Discuss questions about EDUNIX 2.6 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















