
ECHINAMED PLUS SPRAY ORAL SPRAY SOLUTION


How to use ECHINAMED PLUS SPRAY ORAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
EchinaMed plus spray, solution for oral spray.
Read the entire leaflet carefully before starting to use this medication
because it contains important information for you.
This medication can be purchased without a prescription. Nevertheless, to obtain the best
results, it must be used properly.
Follow the administration instructions of the medication contained in
this leaflet or as indicated by your doctor or pharmacist.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience adverse effects, consult your doctor, pharmacist, or nurse,
even if it is an adverse effect not described in this leaflet.
- You should consult a doctor if it worsens, or if it does not improve after 7 days of
treatment.
Contents of the leaflet:
- What is EchinaMed plus spray and what is it used for
- What you need to know before starting to use EchinaMed plus spray
- How to use EchinaMed plus spray
- Possible adverse effects
- Storage of EchinaMed plus spray
- Package contents and additional information
1. What is EchinaMed Plus Spray and what is it used for
EchinaMed plus spray is a traditional herbal medicinal product for the relief of pharyngitis
(sore throat) associated with a cold, cooling, and flu, based on its traditional use.
2. What you need to know before starting to use EchinaMed Plus Spray
Do not use EchinaMed plus sprayif you:
- Are allergic to plants of the Asteraceae (Compositae) family, to sage, or to
any of the product's components.
- Suffer from an autoimmune disease (multiple sclerosis, collagenosis)
- Suffer from conditions that reduce your resistance to infection.
- Are undergoing treatment with immunosuppressants (oncological treatments with
cytostatics or by transplant)
- Suffer from diseases characterized by a low white blood cell count
such as agranulocytosis or leukemia.
Warnings and precautions
- If during the use of the medication, the symptoms worsen or there is a high fever, you
should consult a doctor or pharmacist.
- There is a possible risk of anaphylactic reaction in atopic patients. If you suffer from
urticaria, atopic dermatitis, or asthma, you should consult your doctor before using
this medication.
- This medication contains 40-47% V/V of ethanol, equivalent to 163mg per
dose (81mg per spray), equivalent, for example, to: 3.7 ml of beer per dose (1.85 ml of beer per spray), equivalent, for example, to: 1.5 ml
of wine per dose (0.77 ml of wine per spray)
- It is harmful to alcoholics and should be monitored during pregnancy, breastfeeding,
in children, and in high-risk groups such as patients with liver disease or epileptics.
- This medication contains sorbitol. If your doctor has told you that you have
intolerance to certain sugars, you should consult your doctor before using this
medication.
- This medication contains sucrose. Patients with fructose intolerance, glucose or galactose malabsorption, or sacarase-isomaltase insufficiency,
should not take this medication.
- This medication contains soy lecithin and should not be used by patients with a known
allergy to peanuts or soy.
Children and adolescents
It should not be administered to minors under 18 years of age.
Use of EchinaMed plus spray with other medications:
There is no evidence that extracts of echinacea or sage interact with other medications.
Pregnancy and breastfeeding
As a precautionary measure and in the absence of sufficient data, use during pregnancy and breastfeeding is not recommended without medical supervision.
If you are pregnant or think you may be pregnant, consult your doctor before taking this medication. The consumption of medications during pregnancy can be hazardous to the embryo or fetus and should be monitored by your doctor.
Driving and using machinery:
No studies have been conducted on the effects of EchinaMed plus spray on the ability to drive vehicles and operate machinery. However, based on its activity, it is unlikely that EchinaMed plus spray will alter this ability.
3. How to use EchinaMed Plus Spray
Follow the administration instructions of the medication contained in this leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your pharmacist or doctor.
The recommended dose is:
Adults and elderly: 2 sprays, 6 to 10 times a day.
Start treatment with the first symptoms.
At the end of this leaflet, you can find the instructions for assembling the sprayer.
EchinaMed plus spray is a medication for exclusive buccopharyngeal use.
Do not use more than the recommended dose.
Duration of use
Do not use EchinaMed plus spray continuously for more than 2 weeks.
If symptoms persist for more than 7 days, you should consult a doctor or pharmacist.
Do not take a double dose to make up for forgotten doses.
If you take more EchinaMed plus spray than you should:
In case of excessive intake, symptoms can be expected according to the amount of ethanol (alcohol) ingested
In case of overdose or accidental ingestion, go to a medical center or consult the Toxicology Information Service, phone 91 562 0420, indicating the medication and the amount used.
4. Possible adverse effects
Like all medications, EchinaMed plus spray can have adverse effects, although not all people experience them.
Interrupt treatment and consult your doctor if allergic reactions occur
such as:
- Rash
- Urticaria
- Inflammatory skin disorders (Stevens-Johnson syndrome)
- Swelling of the skin due to fluid
- Swelling of the face (Quincke's edema)
- Reduced airflow to the lungs (bronchospasm)
- Asthma and severe allergic reactions (anaphylactic shock)
Echinacea can trigger allergic reactions in atopic patients.
If you experience adverse effects, consult your doctor or pharmacist, even if it is an adverse effect that does not appear in this leaflet.
Reporting of adverse effects:
If you experience any type of adverse effect, consult your doctor or pharmacist
or nurse, even if it is a possible adverse effect that does not appear in this leaflet. You can also report it directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of EchinaMed Plus Spray
Keep this medication out of sight and reach of children.
Store at room temperature.
Do not use EchinaMed plus spray after the expiration date indicated on the packaging.
Medications should not be thrown away in drains or trash. Ask your pharmacist how to dispose of the packaging and medications that are no longer needed. This will help protect the environment.
6. Package contents and additional information
1 ml of EchinaMed plus spray solution contains as active ingredients:
- 863.3 mg of extract from the fresh aerial part of Echinacea purpurea
- 45.5 mg of extract from the fresh root of Echinacea purpurea
- 430 mg of extract from the fresh leaf of Salvia officinalis
The other components are excipients:
Sucrose laurate (vegetable origin)
Soy lecithin (vegetable origin)
94% ethanol (m/m)
Peppermint oil
Sorbitol (liquid)
Appearance of the product and package contents
EchinaMed plus is a translucent liquid, brown to yellowish-green with
a minty aroma and a fresh, slightly bitter, alcoholic taste.
It is presented in bottles with 30 ml
For buccopharyngeal application.
Instructions for assembling the sprayer
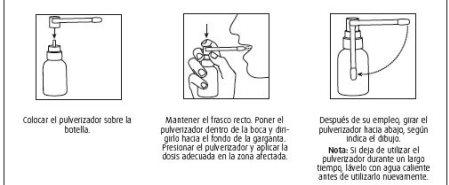
Marketing authorization holder and manufacturer
Bioforce España A.Vogel, S.A.
Platón 6,
08021-Barcelona
Spain
Tel 93 201 99 22
Fax 93 209 03 19
Manufacturer
- VOGEL B.V.
J.P. Broekhovenstraat 16, Elburg
Netherlands
Date of the last revision of this leaflet: January 2020
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)
http://www.aemps.gob.es/
- Country of registration
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ECHINAMED PLUS SPRAY ORAL SPRAY SOLUTIONDosage form: BUCCAL/SUCKING TABLET, 8.75 mgActive substance: flurbiprofenManufacturer: Alfasigma España, S.L.Prescription not requiredDosage form: BUCCAL/SUCKING TABLET, 8.75 mgActive substance: flurbiprofenManufacturer: Alfasigma España, S.L.Prescription not requiredDosage form: BUCCAL/SUCKING TABLET, 8.75 mgActive substance: flurbiprofenManufacturer: Laboratorios Cinfa S.A.Prescription not required
Online doctors for ECHINAMED PLUS SPRAY ORAL SPRAY SOLUTION
Discuss questions about ECHINAMED PLUS SPRAY ORAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















