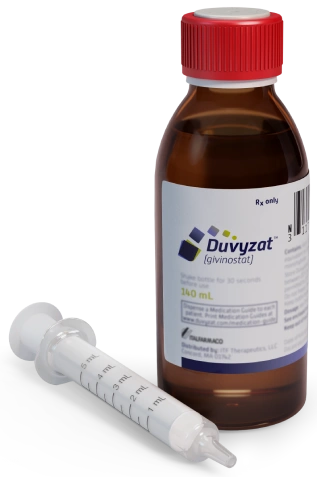
DUVYZAT 8.86 mg/mL ORAL SUSPENSION

How to use DUVYZAT 8.86 mg/mL ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Duvyzat 8.86 mg/ml Oral Suspension
givinostat
This medication is subject to additional monitoring, which will make it easier to detect new information about its safety. You can help by reporting any side effects you may have. The last part of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Duvyzat and what is it used for
- What you need to know before you take Duvyzat
- How to take Duvyzat
- Possible side effects
- Storing Duvyzat
- Contents of the pack and other information
1. What is Duvyzat and what is it used for
Duvyzat contains the active substance givinostat, which is used to treat Duchenne muscular dystrophy (DMD) in patients aged 6 years and older who can walk and are being treated with corticosteroids.
The cause of DMD is mutations in the DMDgene. These changes in the gene compromise the function of muscle cells and lead to progressive muscle degeneration. By blocking the activity of HDAC enzymes (histone deacetylases) in muscle cells, Duvyzat prevents muscle degeneration.
2. What you need to know before you take Duvyzat
Do not take Duvyzat
- if you (or your child) are allergic to givinostat or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before you start taking Duvyzat.
Duvyzat reduces the number of blood cells, especially platelet count, which are responsible for blood clotting (a condition known as thrombocytopenia).
Your doctor will perform blood tests to check platelet levels before treatment and periodically throughout treatment with Duvyzat.
Your doctor may reduce the dose you have been prescribed to increase platelet count or stop treatment with Duvyzat if thrombocytopenia persists.
If you experience any unexpected bleeding, inform your doctor.
Duvyzat may be associated with an increase in blood fat (triglyceride) levels. Before you start treatment with Duvyzat, your doctor will perform blood tests, as well as periodically during treatment, to check triglyceride levels.
If triglyceride levels in the blood remain high, your dose of givinostat may be reduced.
If, despite dietary control measures and dose reduction of the medicine, blood fat (triglyceride) levels do not decrease, your doctor may stop treatment.
During treatment with Duvyzat, you may experience diarrhea and vomiting.
Your doctor may adjust the dose of Duvyzat based on the severity of diarrhea or stop treatment if diarrhea and vomiting do not improve.
Your doctor may consider using medications to treat vomiting and diarrhea and prevent excessive fluid loss.
High doses of Duvyzat (5 times the recommended dose) may cause an irregular heartbeat. If there is an increased risk of an abnormal heartbeat, abnormal mineral concentrations, or you are taking other medications at the same time, your doctor will consider whether you can use Duvyzat.
If you have a pre-existing heart condition or are taking medications that can cause an irregular heartbeat, your doctor may check your heart function before starting treatment with Duvyzat.
Your doctor may consider stopping treatment with Duvyzat if they find that your heart is beating irregularly.
If any of the above conditions occur, contact your doctor, who may stop treatment with Duvyzat.
Other medicines and Duvyzat
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
Duvyzat may increase the risk of side effects caused by other medicines by increasing the amount of these medicines in the blood. Some of these medicines include:
- carbamazepine, phenytoin (a medicine used to treat epilepsy)
- amitriptyline (a medicine used to treat low mood and depression)
- digoxin (a medicine used to treat heart failure and irregular heartbeat)
- metformin (a medicine to control type 2 diabetes)
- amiloride (a medicine to treat high blood pressure)
- histamine 2 receptor antagonists (a medicine used to treat gastroduodenal ulcers and common heartburn)
Caution is recommended when administering Duvyzat with medicines known to cause abnormal heartbeats.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. If you become pregnant during treatment with Duvyzat, consult your doctor immediately. Duvyzat must not be used during pregnancy or breastfeeding.
Driving and using machines
This medicine may cause dizziness or fatigue. If you feel dizzy or tired, do not drive or use machines.
Duvyzat contains sorbitol, sodium benzoate, and sodium
Sorbitol:
This medicine contains 400 mg of sorbitol in each milliliter.
Sorbitol is a source of fructose. If you (or your child) have hereditary fructose intolerance (HFI), a rare genetic disorder, you must not take this medicine. Patients with HFI cannot break down fructose, which can cause serious side effects.
Sodium benzoate:
This medicine contains 4.4 mg of sodium benzoate in each milliliter.
Sodium benzoate may increase the risk of jaundice (yellowing of the skin and eyes) in newborns (up to 4 weeks of age).
Sodium:
This medicine contains less than 23 mg of sodium (1 mmol) per milliliter; it is essentially
"sodium-free).
3. How to take Duvyzat
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, check with your doctor or pharmacist.
Duvyzat should be taken by mouth using the oral syringe. It should be taken twice a day. The recommended dose of Duvyzat depends on your body weight, as shown in Table 1.
Table 1. Recommended dose
Weight (kg) | Volume of Duvyzat oral suspension to be taken twice a day |
Between ≥ 15 and < 20 | 2.5 ml |
Between ≥ 20 and < 40 | 3.5 ml |
Between ≥ 40 and < 60 | 5.0 ml |
≥ 60 | 6.0 ml |
If the prescribed dose is greater than 5 ml per dose, you can use the same oral syringe more than once.
If certain symptoms appear (see Warnings and precautions), your doctor may need to reduce the dose (see Table 2):
- decreased platelet count
- moderate or severe diarrhea (more than 4 bowel movements per day)
- high blood fat levels
Table 2. First dose reduction
Weight (kg) | Volume of Duvyzat oral suspension to be taken twice a day |
Between ≥ 15 and < 20 | 2.0 ml |
Between ≥ 20 and < 40 | 2.5 ml |
Between ≥ 40 and < 60 | 3.5 ml |
≥ 60 | 4.5 ml |
If the above anomalies do not improve, your doctor may further reduce the dose (see Table 3).
Table 3. Second dose reduction
Weight (kg) | Volume of Duvyzat oral suspension to be taken twice a day |
Between ≥ 15 and < 20 | 1.5 ml |
Between ≥ 20 and < 40 | 2.0 ml |
Between ≥ 40 and < 60 | 3.0 ml |
≥ 60 | 4.0 ml |
If these anomalies persist or if you experience an irregular heartbeat, your doctor may consider stopping treatment with Duvyzat.
Method of administration
Duvyzat is administered orally.
The oral suspension should be shaken by hand about 40 times for at least 30 seconds, turning the bottle continuously upside down until the oral suspension is well mixed and has a uniform appearance.
The suspension is dosed with the graduated oral syringe.
Important information about dosing Duvyzat:
Talk to your doctor or pharmacist to show you how to measure your prescribed dose.
- Take Duvyzat as your doctor has told you (see Tables 1, 2, and 3).
- The recommended dose of Duvyzat is taken by mouth twice a day.
- Take Duvyzat as it is presented (do not dilute with water or other liquids).
- Take Duvyzat with food to reduce the bitter taste of givinostat.
- Always take Duvyzat with the oral syringe (5 ml) provided with the medicine.
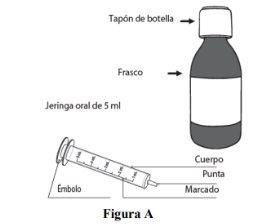
For first use of the bottle: remove the Duvyzat bottle and the 5 ml oral syringe from the box (see Figure A). |
|
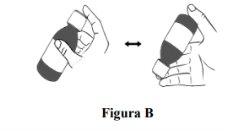
Step 1. Check that the bottle is properly closed and shake it about 40 times for at least 30 secondsturning it continuously upside down (see Figure B). When Duvyzat oral suspension is well mixed and has a uniform appearance, stop shaking. |
|
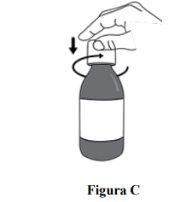
Step 2. Open the bottle by pressing the cap and turning it to the left (counterclockwise) (see Figure C). Do not remove the cap from the bottle. |
|
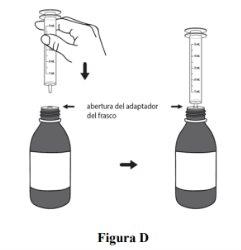
Step 3. For first use:take the oral syringe provided for use and insert the tip of the oral syringe into the adapter opening of the bottle (see Figure D). For subsequent uses:take the oral syringe provided for use, press the plunger to the end (to remove air), and insert the tip of the oral syringe firmly into the adapter opening of the bottle (see Figure D). |
|
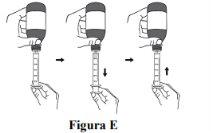
Step 4. While the oral syringe is in place, turn the bottle upside down. Slowly pull the plunger to withdraw a small amount of the suspension. Then, press the plunger to the end to remove any air bubbles that may be present (see Figure E). |
|
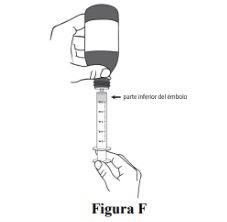
Step 5. Slowly press the plunger down until the edge of the plunger is at the same level as the marks on the oral syringe of the prescribed dose of Duvyzat (see Figure F). If the prescribed dose is greater than 5 ml, you will need to use the same oral syringe more than once. |
|
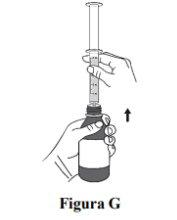
Step 6. With the plunger in the same position, put the bottle upright and carefully place it on a flat surface. Remove the oral syringe by gently turning it or pulling it out of the bottle adapter. Do nothold the oral syringe by the plunger because it may come off (see Figure G). Take or administer Duvyzat immediately after withdrawal with the oral syringe. Do notkeep the oral syringe filled. |
|
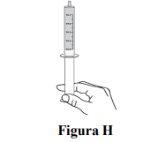
Step 7. Check that you have withdrawn the prescribed volume (ml) of Duvyzat in the oral syringe (see Figure H). In Figure H, an example of a 5 ml dose is shown. Your dose may have a different volume. |
|
Step 8. To take a dose of Duvyzat, the child or adult must be sitting upright with their back straight. Place the tip of the oral syringe against the inside of the cheek. Gently press the plunger until the end until there is no more medicine inside the oral syringe. If your prescribed dose is greater than 5 ml, repeat Steps 3 to 8 to administer the remaining dose volume. | |
Step 9. After use, put the cap back on the bottle and turn it to the right (clockwise) to close the bottle (see Figure I). |
|
Step 10. Wash the oral syringe with water and let it dry. Keep the oral syringe in a clean and dry place. |
If you take more Duvyzat than you should
If you take more than the prescribed dose of Duvyzat, contact your doctor or hospital.
Your doctor will decide on the care you should receive, which may include monitoring your heart activity.
If you forget to take Duvyzat
It is important to take the correct dose.
If you forget a dose, take the next one when it is scheduled. Do not take a double dose to make up for forgotten doses.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
If you stop treatment with Duvyzat
Do not stop taking Duvyzat without talking to your doctor first.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
After taking Duvyzat, you may experience one or more of the following side effects:
Very common side effects (may affect more than 1 in 10 people):
- stomach pain (abdomen)
- reduced platelet count (thrombocytopenia)
- diarrhea
- high blood fat levels (hypertriglyceridemia)
- fever (pyrexia)
- vomiting
Common side effects (may affect up to 1 in 10 people):
- anxiety
- constipation
- decreased appetite
- dizziness
- skin redness (erythema)
- fatigue
- diarrhea and vomiting (gastroenteritis)
- blood accumulation under the skin (hematoma)
- increased levels of thyroid-stimulating hormone or thyrotropin (TSH) in the blood
- joint pain (arthralgia)
- muscle pain (myalgia)
- muscle weakness
- rash
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Duvyzat
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and bottle after "EXP". The expiry date is the last day of the month shown.
Once opened, use within 60 days.
After 60 days from the first opening of the bottle, discard any unused Duvyzat.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Packaging Contents and Additional Information
Duvyzat Composition
The active ingredient is givinostat.
Each milliliter of oral suspension contains 8.86 mg of givinostat (in the form of monohydrate hydrochloride).
The other components are polysorbate 20 (E-432), glycerol (E-422), tragacanth gum (E-413), sodium benzoate (E-211), peach flavor (natural flavorings, flavorings, propylene glycol [E-1520]), cream flavor (natural flavorings, flavorings, propylene glycol [E-1520]), sodium saccharin (E-954), liquid sorbitol (E-420), tartaric acid (E-334), sodium hydroxide (E-524), and purified water.
Product Appearance and Packaging Contents
Duvyzat is a white to off-white or pale pink oral suspension.
Packaging of a 140 ml bottle.
The bottle is packaged with a 5 ml graduated oral syringe. The oral syringe is graduated from 1 to 5 ml, with increments of 0.5 ml.
Marketing Authorization Holder
Marketing Authorization Holder
Italfarmaco S.p.A.
Viale F. Testi, 330
20126 Milan
Italy
Manufacturer
Italfarmaco S.A.
San Rafael, 3
28108 Alcobendas (Madrid)
Spain
or
Italfarmaco S.p.A.
Viale F. Testi, 330
20126 Milan
Italy
Scan the code with a mobile device to obtain the leaflet in different languages.

Or visit the URL https://www.duvyzat.eu
Date of the last revision of this leaflet:{MM/AAAA}.
This medicinal product has been authorized with a «conditional approval». This approval means that more information on this medicinal product is expected. The European Medicines Agency will review the new information on this medicinal product at least once a year and this leaflet will be updated as necessary.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DUVYZAT 8.86 mg/mL ORAL SUSPENSIONDosage form: ORAL SOLUTION/SUSPENSION, 0.75 mg/mlActive substance: risdiplamManufacturer: Roche Registration GmbhPrescription requiredDosage form: TABLET, 5 mgActive substance: risdiplamManufacturer: Roche Registration GmbhPrescription requiredDosage form: INJECTABLE, 20 mgActive substance: hyaluronic acidManufacturer: Laboratorios Fidia Farmaceutica S.L.Prescription required
Online doctors for DUVYZAT 8.86 mg/mL ORAL SUSPENSION
Discuss questions about DUVYZAT 8.86 mg/mL ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions