
DURATIL 20 micrograms/80 microliters Injectable Solution in Pre-filled Pen

How to use DURATIL 20 micrograms/80 microliters Injectable Solution in Pre-filled Pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Duratil20 micrograms/80 microliters solution for injection in pre-filled pen EFG
Teriparatide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Duratil and what is it used for
- What you need to know before you use Duratil
- How to use Duratil
- Possible side effects
- Storage of Duratil
- Contents of the pack and other information
1. What is Duratil and what is it used for
Duratil contains the active substance teriparatide, which is used to increase bone strength and reduce the risk of fractures by stimulating bone formation.
This medicine is used for the treatment of osteoporosis in adults. Osteoporosis is a disease that makes your bones waste away and become fragile. This disease is especially common in women after menopause, but it can also occur in men. Osteoporosis is also common in patients treated with corticosteroids.
2. What you need to know before you use Duratil
Do not use Duratil
- If you are allergic to teriparatide or any of the other ingredients of this medicine (listed in section 6).
- If you have high levels of calcium (pre-existing hypercalcaemia).
- If you have severe kidney problems.
- If you have ever been diagnosed with bone cancer or other types of cancer that have spread (metastasized) to your bones.
- If you have certain bone diseases. If you have a bone disease, consult your doctor.
- If you have high levels of alkaline phosphatase in your blood without apparent reason, which could indicate that you have Paget's disease of the bone (a disease with abnormal bone changes). If you are not sure, consult your doctor.
- If you have received radiation therapy that may have affected your bones.
- If you are pregnant or breastfeeding.
Warnings and precautions
Duratil may cause an increase in the amount of calcium in your blood or urine.
Consult your doctor or pharmacist before starting or while using Duratil:
- If you continuously have nausea, vomiting, constipation, low energy, or muscle weakness, tell your doctor. These may be symptoms of too much calcium in your blood.
- If you have kidney stones or have a history of kidney stones.
- If you have kidney problems (moderate renal insufficiency), you should tell your doctor.
Some patients, after the first doses, experience dizziness or an increased heart rate. For the first doses, use Duratil in a place where you can sit or lie down immediately if you feel dizzy.
The recommended treatment time of 24 months should not be exceeded.
Duratil should not be used in growing adults.
Children and adolescents
Duratil should not be used in children and adolescents (under 18 years of age).
Other medicines and Duratil
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, as interactions may occasionally occur (e.g., digoxin/digitalis, a medicine used to treat heart diseases).
Pregnancy and breastfeeding
Do not use Duratil if you are pregnant or breastfeeding. If you are a woman of childbearing age, you should use effective contraceptive methods during treatment with Duratil. If you become pregnant, treatment with Duratil should be discontinued. Consult your doctor or pharmacist before using any medicine.
Driving and using machines
Some patients may feel dizzy after a Duratil injection. If you feel dizzy, do not drive or use machines until you feel better.
Important information about some excipients of Duratil
This medicine contains less than 23 mg of sodium (1 mmol) per dose unit; it is essentially "sodium-free".
3. How to use Duratil
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is 20 micrograms (corresponding to 80 microliters) administered once a day by subcutaneous injection in the thigh or abdomen. To help you remember to inject your medicine, inject it at the same time every day.
Before using the pen for the first time, you need to prepare it. Please consult the User Manual.
Inject Duratil every day for as long as your doctor prescribes it. The total duration of treatment with Duratil should not exceed 24 months. You should not receive more than one 24-month treatment cycle with Duratil in your lifetime. Duratil can be injected at mealtime.
Consult the User Manual included in the package with instructions on how to use the Duratil pen. The pen can be used with insulin injection needles. Some product presentations include the number of injection needles needed for 28 injections (plus 2 additional spare needles) (see Section 6).
The injection of Duratil should be performed shortly after removing the pen from the refrigerator, as indicated in the User Manual. Return the pen to the refrigerator immediately after use. You should use a new needle for each injection and discard it after each use. Do not store the pen with the needle attached. Never share your Duratil pen with others.
Your doctor may recommend taking calcium and vitamin D with Duratil. Your doctor will indicate how much you should take each day.
Duratil can be used with or without food.
If you use more Duratil than you should
If you have accidentally administered more Duratil than prescribed, consult your doctor or pharmacist. The effects that could be expected from an overdose include nausea, vomiting, dizziness, and headache. In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget or cannot inject Duratil at the usual time,do it as soon as possible on the same day. Do not administer a double dose to make up for forgotten doses. Do not inject more than once on the same day. Do not try to make up for the forgotten dose.
If you stop treatment with Duratil
If you are thinking of stopping treatment with Duratil, please consult your doctor. Your doctor will advise and decide how long you should be treated with Duratil.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most common side effects are pain in the limbs (very common, may affect more than 1 in 10 patients), discomfort, headache, and dizziness (common). If you feel dizzy after an injection, sit or lie down until you feel better. If it does not improve, consult your doctor before continuing treatment. Cases of fainting associated with the use of Duratil have been reported.
If you experience discomfort such as redness of the skin, pain, swelling, itching, bruising, or slight bleeding around the injection site (common), these should disappear within a few days or weeks. If not, inform your doctor as soon as possible.
Some patients may have experienced allergic reactions immediately after injection, consisting of difficulty breathing, swelling of the face, skin rash, and chest pain (rare frequency). In rare cases, severe and potentially life-threatening allergic reactions, including anaphylaxis, may occur.
Other side effects are:
Common: may affect up to 1 in 10 patients
- Increased levels of cholesterol in the blood
- Depression
- Neuralgic pain in the leg
- Feeling of fainting
- Feeling that everything is spinning
- Irregular palpitations
- Breathing difficulties
- Increased sweating
- Muscle cramps
- Lack of energy
- Fatigue
- Chest pain
- Low blood pressure
- Heartburn (pain or burning sensation just below the breastbone)
- Vomiting
- Hernia of the tube that carries food to your stomach
- Low hemoglobin or low red blood cell count (anemia)
Uncommon: may affect up to 1 in 100 patients
- Increased heart rate
- Abnormal heart sound
- Shortness of breath
- Hemorrhoids (piles)
- Accidental loss or leakage of urine
- Increased need to urinate
- Weight gain
- Kidney stones
- Pain in the muscles and joints. Some patients have experienced severe back cramps or pain and had to be hospitalized.
- Increased levels of calcium in the blood
- Increased levels of uric acid in the blood
- Increased levels of an enzyme called alkaline phosphatase
Rare: may affect up to 1 in 1,000 patients
- Reduced kidney function, including kidney failure
- Swelling, mainly in the hands, feet, and legs
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Duratil
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date that appears on the packaging and the pen after CAD. The expiry date is the last day of the month indicated.
Cover the pen with the cap after each use (due to the light sensitivity of the injectable solution).
Duratil should always be stored in the refrigerator (between 2°C and 8°C). Once opened, Duratil can be stored at temperatures up to 25°C for a maximum of 7 days when no refrigeration devices are available. After this period, the medicine should be returned to the refrigerator and used within 28 days after the first injection. The Duratil pen should be discarded if it has been out of the refrigerator at temperatures up to 25°C for more than 7 days.
Do not freeze. Avoid placing the pens near the freezer compartment of the refrigerator to prevent freezing. Do not use Duratil if it is or has been frozen.
Each pen should be discarded properly after 28 days, even if it is not completely empty.
Duratil contains a clear and colorless solution. Do not use Duratil if it has solid particles or if the solution is cloudy or discolored.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Container Contents and Additional Information
Duratil Composition
- The active ingredient is teriparatide. Each milliliter of injectable solution contains 250 micrograms of teriparatide.
- The other components are glacial acetic acid, sodium acetate (anhydrous), mannitol, metacresol, and water for injectable preparations. Additionally, a hydrochloric acid and/or sodium hydroxide solution may have been added to adjust the pH.
Product Appearance and Container Contents
Duratil is a clear and colorless solution. It is presented in a cartridge included in a disposable pre-filled pen. Each pen contains 2.4 ml of solution sufficient for 28 doses. The product is available in containers that contain:
- 1 pen with 28 injection needles (plus 2 additional spare needles)
- 1 pen
- 3 pens
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
GP-PHARM, S.A.
Polígono Industrial Els Vinyets-Els Fogars, Sector 2, Carretera Comarcal C-244, Km 22
08777 Sant Quintí de Mediona
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
- Spain: Duratil 20 micrograms/80 microliters solution for injection in pre-filled pen EFG
Date of the Last Revision of this Leaflet:April 2025
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
USER MANUAL FOR THE PEN
User Manual
Instructions for Use
General Description of the Pen Parts
Duratil is a medicinal product supplied in a pen. The pen contains medicinal product for one injection per day for 28 consecutive days.
Use a new needle for each injection.
The needles may not be supplied with the pen; in this case, the pen can be used with insulin injection needles. Consult your doctor or pharmacist to determine the most suitable needle for you.
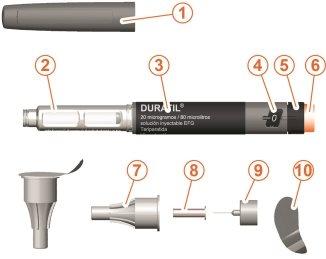
- Pen cap
- Cartridge with the medicinal product
- Label
- Dose window
- Dose selector
- Dose button
- External needle protector
- Internal needle protector
- Needle
- Sealing film
For Your Safety
Important Information
- Read the instructions for use in their entirety. Follow all instructions carefully.
- Read the leaflet provided with the pen.
- If you have questions, contact your doctor, pharmacist, or caregiver.
Prevention of Infectious Diseases
- Do not share your pen, as this could lead to the transmission of infectious diseases.
- Use a new sterile needle for each injection. Used needles pose a risk of transmitting infectious diseases.
Using the Pen
- Check the pen label when you remove the pen from the refrigerator. Make sure you are using the correct medicinal product.
- Check the expiration date; do not use the pen if the expiration date has been exceeded.
- Check the medicinal product: it should be transparent, colorless, and free of particles.
- Do not transfer the medicinal product to a syringe. Duratil must be administered using this pen.
- Do not use the pen after 28 injections. Note the first day of injection in the Injection Diary on the back of these instructions for use. Calculate the date for the 28th injection using a calendar and also note this date in the Injection Diary.
- The use of the pen is not recommended for blind or visually impaired persons without the assistance of a support person.
Storage
- Keep the pen in the refrigerator, preferably in the door compartment.
- Keep the pen and needles out of the reach of children.
Troubleshooting
- If you encounter a problem with the injection, do not administer a second injection on the same day.
- Read the section "What to do if..." in these Instructions for Use.
- Do not use the pen if it is damaged.
- Use the pen only if the medicinal product is transparent, colorless, and free of particles.
- If you cannot resolve the problem yourself or if you are unsure, contact your doctor, pharmacist, or caregiver.
Preparing the Pen Before the First Injection
Before the FIRST injection, you must prepare your pen as described below.
You only need to perform this step once. You do NOT need to repeat this procedure for the second injection or any subsequent injections.
Attaching the Needle
- Remove the pen cap.
- Take a new needle and remove the sealing film from the external needle protector.
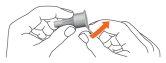
- Place the needle with the external needle protector onto the pen. Screw the external needle protector clockwise until it stops.
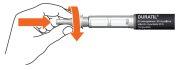
- Remove the external needle protector and set it aside for later use.

Setting the Dose
- Turn the dose selector until it stops. Make sure the number "80" is completely visible and centered in the dose window with the white mark aligned in the dose window slot.

- Remove the internal needle protector and discard it.
- Hold the pen with the needle facing upwards. Press the dose button until it stops and hold it for 5 seconds (see the figure on the next page).
- Confirm the dose. Make sure the number "0" is completely visible and centered in the dose window, the white mark is aligned in the dose window slot, and the engraved marks on the dose selector and the pen body are aligned.

Removing the Needle
Always remove the needle immediately after using the pen.
- Carefully insert the needle into the external needle protector that you set aside in step 4. Do not touch the needle to avoid pricking yourself.

- Unscrew the needle by turning the external needle protector counterclockwise and remove the needle from the pen.

- Discard the needle with the external needle protector in a puncture-proof container that you can obtain from your pharmacy or doctor.
Maintaining the Injection Diary
- Note the current date and the date of the 28th injection in the Injection Diary on the back of these instructions for use.
Now your pen is ready for the first and all subsequent injections as described in the following section:
Injecting Duratil
Prepare for Injection
- Wash your hands before each injection.
- Prepare the injection site (on the thigh or abdomen) according to the instructions of your doctor, pharmacist, or caregiver.
Use a new needle for each injection because a new needle is sharp and allows for a virtually painless injection. A used needle poses a risk of being obstructed or contaminated.
Attaching the Needle
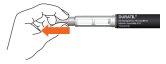
- Remove the pen cap.
- Take a new needle and remove the sealing film from the external needle protector.
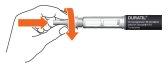
- Place the needle with the external needle protector onto the pen. Screw the external needle protector clockwise until it stops.
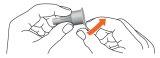
- Remove the external needle protector and set it aside for later use.

Setting the Dose
- Turn the dose selector until it stops. Make sure the number "80" is completely visible and centered in the dose window with the white mark aligned in the dose window slot.

- Remove the internal needle protector and discard it.
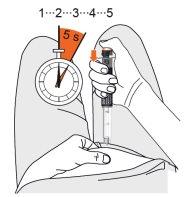
- Gently pinch a skin fold on the thigh or abdomen.
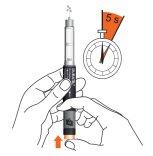
- Insert the needle preferably at a 90-degree angle into the prepared injection site. Press the dose button until it stops and hold it for 5 seconds. Count slowly to 5.
- Remove the needle from the skin.
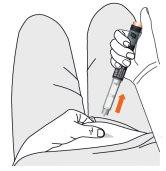
- Confirm administration of the dose. Make sure the number "0" is completely visible and centered in the dose window, the white mark is aligned in the dose window slot, and the engraved marks on the dose selector and the pen body are aligned.

Removing the Needle
Always remove the needle immediately after using the pen.
- Carefully insert the needle into the external needle protector that you set aside in step 4. Do not touch the needle to avoid pricking yourself.

- Unscrew the needle by turning the external needle protector counterclockwise and remove the needle from the pen.

- Discard the needle with the external needle protector in a puncture-proof container that you can obtain from your pharmacy or doctor.
- Put the pen cap back on the pen.
Storing the Pen
Do not store the pen with a needle attached. This can cause air bubbles to form in the medication cartridge. Always cover the pen with the pen cap.
Remove the pen from the refrigerator only when you are about to use it. Store the pen in the refrigerator, preferably in the door compartment. Do not store the pen near the back wall of the refrigerator or in the freezer. The medicinal product becomes unusable if it freezes.
If your pen has not been stored in the refrigerator for a long time, do not discard it. Put the pen back in the refrigerator and consult your doctor, pharmacist, or caregiver.
Disposable Pen
The pen must be discarded on the day of the last injection (see the Injection Diary). Discard the pen even if there is still medicinal product left in the cartridge.
Discard the pen according to the instructions of your doctor or pharmacist.
Put the pen cap on before discarding it. Do not throw away the pen with the needle attached.
What to Do If…
Air Bubbles in the Cartridge: you can use your pen without any problems.
While Preparing the Pen for the First Use, the Pen Does Not Expel Medicinal Product: repeat the steps described in the "Setting the Dose" section (page 5).
The Dose Button is Blocked or You Have the Impression That You Have Not Injected the Full Dose: do not administer a second injection on the same day. Continue with your regular injection the next day. Make sure to turn the dose selector until it stops and that the number "80" is completely visible and centered in the dose window with the white mark aligned in the dose window slot.
Injection Diary |
Date of the first injection: | Day 1 | |
Day 2 | ||
Day 3 | ||
Day 4 | ||
Day 5 | ||
Day 6 | ||
Day 7 | ||
Day 8 | ||
Day 9 | ||
Day 10 | ||
Day 11 | ||
Manufacturer GP-PHARM, S.A. Polígono Industrial Els Vinyets-Els Fogars, Sector 2, Carretera Comarcal C-244, Km 22 08777 Sant Quintí de Mediona Spain | Day 12 | |
Day 13 | ||
Day 14 | ||
Day 15 | ||
Day 16 | ||
Day 17 | ||
Day 18 | ||
Day 19 | ||
Day 20 | ||
Day 21 | ||
Day 22 | ||
Day 23 | ||
Day 24 | ||
Day 25 | ||
Day 26 | ||
Day 27 | ||
Date of the last injection: | Day 28 |
The User Manual was last updated in August 2020.
- Country of registration
- Average pharmacy price252.16 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DURATIL 20 micrograms/80 microliters Injectable Solution in Pre-filled PenDosage form: INJECTABLE, 250 µg/mlActive substance: teriparatideManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE, 20 micrograms/80 microlitersActive substance: teriparatideManufacturer: Theramex Ireland LimitedPrescription requiredDosage form: INJECTABLE, 20/80 µg/mlActive substance: teriparatideManufacturer: Stada Arzneimittel AgPrescription required
Online doctors for DURATIL 20 micrograms/80 microliters Injectable Solution in Pre-filled Pen
Discuss questions about DURATIL 20 micrograms/80 microliters Injectable Solution in Pre-filled Pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











