
Osteoteri
Ask a doctor about a prescription for Osteoteri

How to use Osteoteri
Leaflet included in the packaging: information for the user
Osteoteri, 20 micrograms/80 microliters, solution for injection in a pre-filled pen
Teriparatide
You should carefully read the contents of this leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Osteoteri and what is it used for
- 2. Important information before using Osteoteri
- 3. How to use Osteoteri
- 4. Possible side effects
- 5. How to store Osteoteri
- 6. Contents of the packaging and other information
1. What is Osteoteri and what is it used for
Osteoteri contains the active substance teriparatide, which strengthens bones and reduces the risk of fractures by stimulating bone formation.
Osteoteri is used to treat osteoporosis in adults. The bones of people with osteoporosis become thinner and more fragile. Osteoporosis often occurs in women after menopause, but it can also occur in men. Osteoporosis also often occurs in patients taking glucocorticosteroids.
2. Important information before using Osteoteri
When not to use Osteoteri:
- if you are allergic to teriparatide or any of the other ingredients of this medicine (listed in section 6);
- if you have had high levels of calcium in the blood (pre-existing hypercalcemia);
- if you have severe kidney disease;
- if you have ever had bone cancer or other cancer that has spread to the bone;
- if you have bone diseases. You should inform your doctor if you have bone diseases;
- if you have increased activity of alkaline phosphatase in the blood of unknown cause, as this may indicate Paget's disease (a disease with abnormal bone changes). In case of doubt, you should ask your doctor;
- if you have had radiation therapy involving the bone;
- if you are pregnant or breastfeeding.
Warnings and precautions
Osteoteri may increase the level of calcium in the blood or urine.
Before starting or during treatment with Osteoteri, you should discuss this with your doctor or pharmacist:
- if you experience prolonged nausea, vomiting, constipation, lack of energy, or weakness. This may be a sign of too high a level of calcium in the blood;
- if you have had kidney stones;
- if you have kidney disease (moderate renal impairment).
After a few initial doses of Osteoteri, some patients may experience dizziness or rapid heartbeat. If you experience dizziness with the first doses, you should inject Osteoteri while sitting or lying down.
You should not exceed the recommended 24-month treatment period.
Osteoteri should not be used in adults during the growth period.
Children and adolescents
Osteoteri should not be used in children and adolescents (under 18 years of age).
Osteoteri and other medicines
You should tell your doctor or pharmacist about all medicines you are taking or have recently taken, as they may sometimes interact with Osteoteri (e.g., digoxin or digitalis glycosides used to treat heart conditions).
Pregnancy and breastfeeding
Pregnant or breastfeeding women should not use Osteoteri. Women of childbearing age must use an effective method of contraception during treatment with Osteoteri. If you become pregnant, you should stop using Osteoteri. Before using any medicine, you should consult your doctor or pharmacist.
Driving and using machines
Some patients may experience dizziness after injecting Osteoteri. If you experience dizziness, you should not drive or operate machinery until the dizziness has passed.
Osteoteri contains sodium:
The medicine contains less than 1 mmol (23 mg) of sodium per dose, which means that it is essentially 'sodium-free'.
3. How to use Osteoteri
This medicine should always be used exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
The recommended dose is 20 micrograms (corresponding to 80 microliters) once a day by subcutaneous injection into the thigh or abdomen. To help you remember to use the medicine, injections should be performed at approximately the same time every day.
Before the first use, the pen should be prepared. You should also read the attached instructions for use.
Injections should be performed daily for the period determined by your doctor. The total treatment period with Osteoteri should not exceed 24 months. You can only be treated with Osteoteri for 24 months once in your lifetime.
Osteoteri can be injected at any time of day, with or without food.
You should read the instructions for use included in the packaging, where you can find information on how to use the Osteoteri pen.
No needles are included with the pens. You can use, for example, needles from Becton Dickinson and Company, size 29 to 31 (diameter 0.25-0.33 mm) and length 12.7; 8 or 5 mm.
Injections should be performed shortly after removing the pen from the refrigerator, as described in the instructions for use. The pen should be returned to the refrigerator immediately after use. A new needle should be used for each injection and discarded after use. Never store the pen with the needle attached. Never share the Osteoteri pen with other people.
Your doctor may recommend taking calcium and vitamin D supplements with Osteoteri. In such cases, your doctor will determine the doses of these additional medicines.
Osteoteri can be used with or without food.
What to do if you use more Osteoteri than you should
If you accidentally inject more Osteoteri than you should, contact your doctor or pharmacist.
Expected symptoms that may occur as a result of overdose include nausea, vomiting, dizziness, and headache.
What to do if you miss a dose of Osteoteri
If you forget to inject a dose or are unable to do so at the usual time, you should inject it as soon as possible on the same day. Do not take a double dose to make up for a missed dose. Do not perform more than one injection in a 24-hour period. Do not try to make up for a missed dose.
What to do if you stop using Osteoteri
If you are considering stopping treatment with Osteoteri, you should contact your doctor. Your doctor will advise and decide how long you should use Osteoteri.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Osteoteri can cause side effects, although not everybody gets them.
The most common side effects are: pain in the extremities (very common, may affect more than 1 in 10 people), nausea, headache, and dizziness (common).
If you experience dizziness after injecting Osteoteri, you should sit or lie down until you feel better. If you do not feel better, you should contact your doctor before continuing treatment. Cases of fainting have been reported in association with teriparatide use.
If you experience discomfort symptoms such as redness of the skin, pain, swelling, itching, bruising, or minor bleeding at the injection site (common side effects), they should resolve within a few days or weeks. If not, you should inform your doctor as soon as possible.
In some patients, allergic reactions may occur shortly after injection, such as shortness of breath, facial swelling, rash, and chest pain (rare). In rare cases, severe and potentially life-threatening allergic reactions, including anaphylaxis, may occur.
Other side effects:
- increased blood cholesterol levels
- depression
- leg pain
- weakness
- dizziness
- irregular heartbeat
- shortness of breath
- increased sweating
- muscle cramps
- feeling of lack of energy
- fatigue
- chest pain
- decreased blood pressure
- heartburn (feeling of pain or burning below the breastbone)
- vomiting
- hiatus hernia - a tube that carries food to the stomach
- low hemoglobin or red blood cell count (anemia)
Uncommon: may affect up to 1 in 100 people
- rapid heartbeat
- abnormal heart rhythms
- shortness of breath
- hemorrhoids
- involuntary urination or leakage of urine
- pressure on the bladder
- weight gain
- kidney stones
- muscle and joint pain. Some patients experienced severe muscle cramps or back pain that required hospital treatment.
- increased blood calcium levels
- increased blood uric acid levels
- increased activity of the enzyme alkaline phosphatase
Rare: may affect up to 1 in 1,000 people
- kidney function disorders, including kidney failure
- edema, mainly of the hands, feet, and legs
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, you should tell your doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C,
02-222 Warsaw
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Osteoteri
The medicine should be stored out of sight and reach of children.
Do not use the medicine after the expiry date stated on the carton and pen after EXP. The expiry date refers to the last day of that month.
After use, the pen should be closed with the cap (due to the sensitivity of the injection solution to light). Osteoteri should always be stored in the refrigerator (2°C to 8°C). After the first injection, the medicine can be used for 28 days, provided it is stored in the refrigerator (2°C to 8°C).
Do not freeze Osteoteri. You should avoid placing the pens in the refrigerator near the freezer compartment to prevent freezing of the medicine. Do not use Osteoteri if it has been frozen.
After 28 days, the pen should be discarded, even if it is not completely empty.
Osteoteri is a clear and colorless solution. Do not use Osteoteri if you notice any particles in the solution, if it is cloudy, or if it has changed color.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Osteoteri contains
- The active substance is teriparatide. One milliliter of the solution for injection contains 250 micrograms of teriparatide.
- The other ingredients are: glacial acetic acid, sodium acetate anhydrous, mannitol, metacresol, water for injections, hydrochloric acid (for pH adjustment), sodium hydroxide (for pH adjustment).
What Osteoteri looks like and contents of the pack
Osteoteri is a clear and colorless solution. The medicine is contained in a cartridge located in the pen.
Each pen contains 2.4 ml of solution, which is sufficient for 28 doses.
Pens are available in cardboard boxes containing 1 or 3 pens. Not all pack sizes may be marketed.
Marketing authorization holder
G.L. Pharma GmbH
Schlossplatz 1
8502 Lannach
Austria
Manufacturer
G.L. Pharma GmbH
Schlossplatz 1
8502 Lannach
Austria
GP-PHARM, S.A.
Polígono Industrial Els Vinyets-Els Fogars, Sector 2, Carretera Comarcal C-244, Km 22
08777 Sant Quintí de Mediona
Spain
To obtain more detailed information and information on the names of the medicinal product in other EEA member states, please contact the marketing authorization holder:
G.L. PHARMA POLAND Sp. z o.o.
Al. Jana Pawła II 61/313
01-031 Warsaw, Poland
Tel: 022/ 636 52 23; 636 53 02
[email protected]
Date of last revision of the leaflet:
INSTRUCTIONS FOR USE OF THE PEN
Instructions for use of the pen
Parts of the pen
Osteoteri is a medicine delivered in a pen. The pen contains the medicine to be injected once a day for the next 28 days.
A new needle should be used for each injection. Needles are not supplied with the pen.
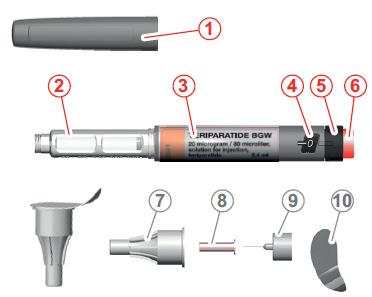
- 1. pen cap
- 2. cartridge
- 3. label
- 4. dosing window
- 5. dose dial
- 6. button
- 7. large outer cap
- 8. small inner cap
- 9. needle
- 10. protective foil
For patient safety
Important information
- You should read the entire instructions for use. You should follow the instructions carefully.
- You should read the contents of the leaflet included with the pen.
- If you have any questions or doubts, you should contact your doctor, pharmacist, or nurse.
Prevention of infectious diseases
- You should not lend the pen to other people, as this may pose a risk of transmitting infectious diseases.
- A new sterile needle should be used for each injection. Used needles pose a risk of transmitting infectious diseases.
Using the pen
- When removing the pen from the refrigerator, you should check the label. You should make sure that you are using the correct medicine.
- You should check the expiry date of the medicine. You should not use the medicine if the expiry date has been exceeded.
- You should check the appearance of the medicine: the solution should be clear, colorless, and free of particles.
- You should not transfer the medicine to another syringe. Osteoteri should be administered using this pen.
- You should not use the pen for more than 28 injections. You should note the date of the first injection in the injection diary attached to the back of these instructions. You should calculate the date of the 28th injection using a calendar and also note it in the injection diary.
- The pen is not intended for use by blind or partially sighted people without assistance from another person.
Storage
- The pen should be stored in the refrigerator, preferably on the door of the refrigerator.
- The pen and needles should be stored out of sight and reach of children.
Troubleshooting
- If you experience difficulties with the injection, you should not perform a second injection on the same day.
- You should read the "What to do if..." section of these instructions.
- You should not use the pen if it is damaged.
- The pen can only be used if the medicine contained in it is clear, colorless, and free of particles.
- If you are unable to cope with the difficulties or have questions, you should contact your doctor, pharmacist, or nurse.
Preparing the pen for first use
Before the FIRST injection, you should prepare the pen as described below.
These steps are performed only once. THERE IS NO NEED to repeat this procedure for the second and subsequent injections.
Attaching the needle
- 1. Remove the cap from the pen.
- 2. Take a new needle and remove the protective foil from the needle cap.
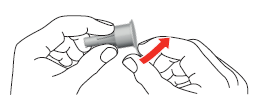
- 3. Attach the needle to the pen using the large outer cap. Turn the cap in the direction of the arrow until it clicks.
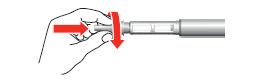
- 4. Remove the large outer cap from the needle and keep it for future use.
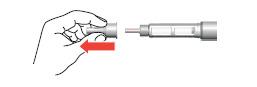
Setting the dose
- 5. Turn the dose dial until it clicks. Make sure that the number "80" is fully visible in the center of the dosing window, on the line of the white marker in the window cutout.
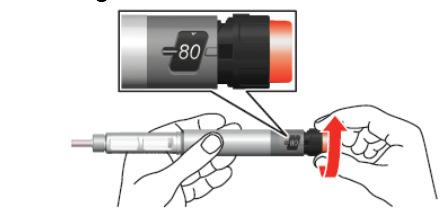
- 6. Remove the small inner cap from the needle and discard it.
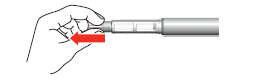
- 7. Hold the pen with the needle facing upwards. Press the dose button until it clicks and hold for 5 seconds. To do this, you should slowly count to five.
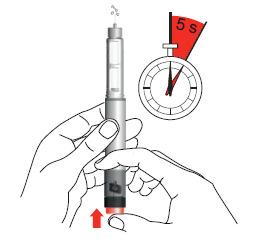
- 8. Confirmation of the dose. You should make sure that the number "0" is fully visible in the center of the dosing window, the white marker is on the line in the window cutout, and the embossed markings on the dose dial and pen body are aligned.

Removing the needle
After preparing the pen, you should always remove the needle immediately, as there is a risk that it may be contaminated.
- 9. Carefully insert the needle into the large outer cap. Do not touch the needle to avoid pricking yourself.
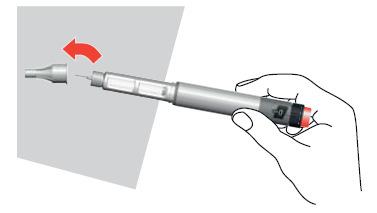
- 10. Remove the needle from the pen by turning the cap in the opposite direction of the arrow and pulling the needle out of the pen.

- 11. Discard the needle with the cap into a safe container for disposal.
Keeping an injection diary
- 12. You should write the date of the first injection and the date of the 28th injection in the injection diary attached to the back of these instructions.
Now the pen is ready for the first and all subsequent injections, as described below.
Injecting Osteoteri
Preparing for injection
- Before each injection, you should wash your hands.
- The injection site (on the thigh or abdomen) should be prepared according to the instructions of your doctor, pharmacist, or nurse.
A new needle should be used for each injection, as an unused needle is sharp and allows for a nearly painless injection. A used needle may be clogged or contaminated.
Attaching the needle
- 1. Remove the cap from the pen.
- 2. Take a new needle and remove the protective foil from the needle cap.
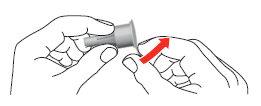
- 3. Attach the needle to the pen using the large outer cap. Turn the cap in the direction of the arrow until it clicks.
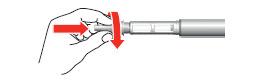
- 4. Remove the large outer cap from the needle and keep it for future use.
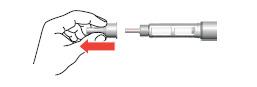
Setting the dose
- 5. Turn the dose dial until it clicks. Make sure that the number "80" is fully visible in the center of the dosing window, on the line of the white marker in the window cutout.
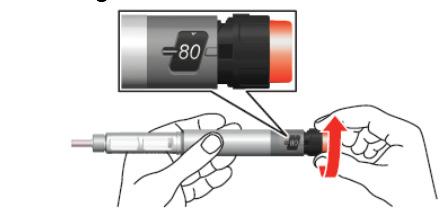
- 6. Remove the small inner cap from the needle and discard it.
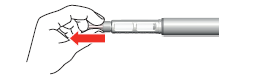
- 7. Gently hold the skin fold on the thigh or abdomen
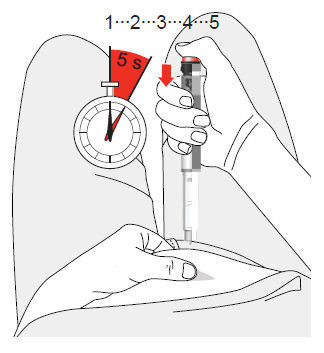
- 8. Insert the needle at a 90-degree angle into the prepared injection site. Press the button until it clicks and hold for 5 seconds. To do this, you should slowly count to five.
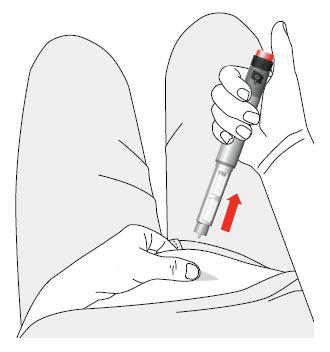
- 9. Remove the needle from the body.
- 10. Confirmation of the dose. You should make sure that the number "0" is fully visible in the center of the dosing window, the white marker is on the line in the window cutout, and the embossed markings on the dose dial and pen body are aligned.

Removing the needle
After using the pen, you should always remove the needle immediately.
- 11. Carefully insert the needle into the large outer cap. Do not touch the needle to avoid pricking yourself.
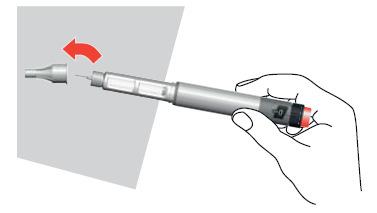
- 12. Remove the needle from the pen by turning the cap in the opposite direction of the arrow and pulling the needle out of the pen.

- 13. Discard the needle with the cap into a safe container for disposal.
- 14. Put the cap back on the pen.
Storing the pen
You should not store the pen with the needle attached, as this may cause air bubbles to form in the cartridge.
You should always put the cap back on the pen.
The pen should be removed from the refrigerator only for use. The pen should be stored in the refrigerator, preferably on the door of the refrigerator.
You should not store the pen near the back wall of the refrigerator or in the freezer. If the pen has been frozen, the medicine is no longer usable.
If the pen has not been stored in the refrigerator for a longer period, you should not discard it.
You should put the pen in the refrigerator and contact your doctor, pharmacist, or nurse.
Disposing of the pen
The pen should be discarded on the day of the last injection (see injection diary).
The pen should be discarded, even if there is still medicine left in the cartridge.
The pen should be discarded according to the instructions of your doctor or pharmacist.
Before disposal, you should put the cap back on the pen.
You should not discard the pen with the needle attached.
What to do if...
Air bubbles in the cartridge:you can use the pen without worrying.
If, during preparation of the pen for first use, no liquid comes out of the pen:
you should repeat the actions described in the "Setting the dose" section on page 5 of these instructions.
If the button is blocked or you feel that you have not injected the full dose:you should not perform a second injection on the same day. You should perform the next injection the following day. You should make sure that the dose dial is turned until it clicks, and the number "80" is fully visible in the center of the dosing window, on the line of the white marker in the window cutout.
Injection diary
| Date of first injection: day 1 day 2 day 3 day 4 day 5 day 6 day 7 day 8 day 9 day 10 day 11 day 12 day 13 day 14 day 15 day 16 day 17 day 18 day 19 day 20 day 21 day 22 day 23 day 24 day 25 day 26 day 27 Date of last injection: day 28 | |
Date of last revision of these instructions
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterG.L. Pharma GmbH GP-PHARM S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OsteoteriDosage form: Tablets, 30 mgActive substance: cinacalcetPrescription requiredDosage form: Tablets, 60 mgActive substance: cinacalcetPrescription requiredDosage form: Tablets, 90 mgActive substance: cinacalcetPrescription not required
Alternatives to Osteoteri in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Osteoteri in Spain
Online doctors for Osteoteri
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Osteoteri – subject to medical assessment and local rules.










