DITRALIA 25,000 IU ORALLY DISINTEGRATING TABLETS

Ask a doctor about a prescription for DITRALIA 25,000 IU ORALLY DISINTEGRATING TABLETS

How to use DITRALIA 25,000 IU ORALLY DISINTEGRATING TABLETS
Introduction
Package Leaflet: Information for the User
Ditralia 25000 IUorodispersible films
colecalciferol (vitamin D3)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Ditralia and what is it used for
- What you need to know before you take Ditralia
- How to take Ditralia
- Possible side effects
- Storing Ditralia
- Contents of the pack and other information
1. What is Ditralia and what is it used for
Ditralia contains colecalciferol (vitamin D3). The main function of vitamin D is to ensure proper intestinal absorption of calcium and to promote adequate mineralization of bones.
Ditralia is indicated for the initial treatment of clinically relevant vitamin D deficiency in adults.
2. What you need to know before you take Ditralia
Do not take Ditralia
- if you are allergic to colecalciferol or any of the other ingredients of this medicine (listed in section 6)
- if you have a particular sensitivity to vitamin D (tissue damage in various organs caused by vitamin D overdose) (hypervitaminosis D)
- if you have high levels of calcium in the blood
- if you have high levels of calcium in the urine, especially if you have kidney stones
- if you have calcium deposits in the kidneys (nephrocalcinosis)
- if you have severely reduced kidney function (severe renal insufficiency)
If you are in any of the above situations, consult your doctor or pharmacist before taking Ditralia.
Warnings and precautions
Consult your doctor or pharmacist before taking Ditralia.
Tell your doctor if you are taking other products that contain vitamin D, such as fortified foods and milk, as vitamin D accumulates in the body and an overdose can cause toxic effects. Ditralia should be used with caution in immobilized patients.
Therefore, it is important not to exceed the recommended dose.
Consult your doctor before taking Ditralia:
- if you have a high tendency to form kidney stones
- if you have a parathyroid hormone imbalance (pseudo-hypoparathyroidism)
If you have any of the following conditions, your doctor will monitor your calcium or phosphate levels in the blood, or your calcium levels in the urine:
- if you have kidney problems
- if you suffer from "sarcoidosis", an immune system disease that can affect the liver, lungs, skin, or lymph nodes
- if you are an elderly person being treated with glycosides or diuretics
- if you are immobilized
Children and adolescents
This medicine is not recommended for children and adolescents under 18 years of age.
Other medicines and Ditralia
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription.
In particular, consult your doctor if you are taking any of the following medicines:
- thiazide diuretics: your calcium levels in the blood will be regularly monitored
- corticosteroids ("steroids", e.g., prednisolone or dexamethasone): your vitamin D dose may need to be increased
- cholestyramine (a medicine to reduce cholesterol) or laxatives (e.g., paraffin oil): reduce vitamin D absorption
- heart medicines (cardiac glycosides): you will be under medical supervision and your ECG and calcium levels in the blood may be monitored
- anticonvulsants (for the treatment of epilepsy; e.g., phenytoin), sleeping medicines (e.g., hydantoin, barbiturics), or primidone: reduce the effects of vitamin D
- rifampicin, isoniazid (antibiotics): may reduce the effectiveness of vitamin D
- actinomycin (a medicine used to treat some types of cancer) and antifungal agents derived from imidazole (e.g., clotrimazole and ketoconazole, medicines used to treat fungal infections): may reduce the effectiveness of vitamin D
- products containing high doses of calcium: increase the risk of high calcium levels in the blood
- products containing magnesium (e.g., antacids): should not be used during treatment with vitamin D due to the risk of high magnesium levels
- products containing high doses of phosphorus: concomitant use with vitamin D may increase the risk of high phosphate levels in the blood
- orlistat (a medicine used to treat obesity, including weight loss): may reduce vitamin D absorption. There should be a 2-hour interval (before and after) between the administration of vitamin D and orlistat.
Taking Ditralia with food and drinks
See section 3 "How to take Ditralia".
Pregnancy, breastfeeding, and fertility
During pregnancy and breastfeeding, high doses of this product should not be used, but lower doses. If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking vitamin D. Vitamin D3 passes into breast milk. This should be taken into account when administering additional vitamin D to the infant. There are no data on the effects of vitamin D3 on fertility. However, it is not expected that normal levels of vitamin D will have negative effects on fertility.
Driving and using machines
Ditralia has no or negligible influence on the ability to drive and use machines.
Ditralia contains orange yellow:
Orange yellow (E110), which may cause allergic reactions.
3. How to take Ditralia
Follow exactly the administration instructions of this medicine given by your doctor. If you are not sure, ask your doctor or pharmacist.
Use in adults
Your doctor will supervise the treatment, as the dose and duration of therapy depend on your 25-hydroxycolecalciferol (25(OH)D) level in the blood, the severity of your disease, and your response to treatment.
Recommended dose: 1 orodispersible film of 25,000 IU per week for the first month. After the first month of treatment, your doctor may consider reducing the dose.
Alternatively, national dosage recommendations for the treatment of vitamin D deficiency can be followed.
Use in children and adolescents
Ditralia is not intended for use in children and adolescents under 18 years of age.
Instructions for use
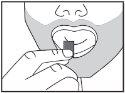
The orodispersible film should be placed in the mouth, on the tongue, and allowed to dissolve before swallowing. It should be taken immediately after removal from the sachet. Ditralia can be taken with or without food.
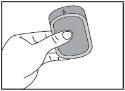
Important: Do not handle the orodispersible film with wet hands.
|
|
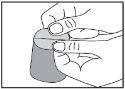
To do this, hold each part with one hand, using your thumb and index finger. |
|
You will see the orodispersible film placed in one of the separated parts of the sachet. |
|
|
|
If you take more Ditralia than you should
If you take an excessive dose of Ditralia, consult your doctor or pharmacist immediately. Excess vitamin D causes an alteration of the calcium cycle in the body. The following symptoms may be experienced: weakness, fatigue, headache, nausea, vomiting, diarrhea (and in later stages, constipation), excessive urine production, urinary calcium, dry mouth, nocturia, intense thirst, loss of appetite, muscle and joint pain, abdominal pain, and arrhythmia.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
Emergency treatment:
Calcitonin, corticosteroid therapy (which prevents intestinal absorption of calcium), abundant hydration, diuretics to increase calcium content in the urine (furosemide), a low-calcium diet. High levels of calcium in the blood require immediate hospitalization.
If you forget to take Ditralia
Do not take a double dose to make up for forgotten doses.
If you stop taking Ditralia
Do not stop treatment unless you experience side effects. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop treatment with Ditralia and consult your doctor immediately
- If you experience any of the following symptoms, as they may be a sign of an allergic reaction (hypersensitivity):
- swelling of the face, lips, tongue, or throat
- difficulty swallowing
- hives
- difficulty breathing
Uncommon side effects (may affect up to 1 in 100 people):
- excess calcium in the blood (hypercalcemia)
- excess calcium in the urine (hypercalciuria)
Rare side effects (may affect up to 1 in 1,000 people):
- pruritus, skin rash, and urticaria
Frequency not known (cannot be estimated from the available data):
- constipation, flatulence, nausea, abdominal pain, diarrhea
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Ditralia
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton after EXP.
Do not store above 30°C.
Store the product in its original packaging to protect it from light.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to the SIGRE collection point at your pharmacy. If you have any further questions on how to dispose of the containers and any unused medicines, ask your pharmacist. This will help protect the environment.
6. Contents of the pack and other information
Composition of Ditralia
The active substance is colecalciferol. Each film contains 625 micrograms of colecalciferol (vitamin D3), equivalent to 25,000 IU.
The other ingredients (excipients) are refined olive oil, purified water, maltodextrin, hydroxypropylbetadex, copovidone, mannitol (E421), glycerol (E422), polysorbate 80 (E433), mono-linoleate glycerol, titanium dioxide (E171), sucralose (E955), orange flavor*, ascorbic acid (E300), all-rac-alpha-tocopherol (E307), orange yellow (E110).
*contains:
Flavor: orange essential oil, terpene-free orange oil, terpene-free lemon oil, terpene-free mandarin oil, ethyl hexanoate, ethyl 2-methylbutyrate, ethyl butyrate, acetaldehyde
Additives: butylhydroxyanisole (E320), citric acid (E330)
Fillers: maltodextrin, arabic gum (E414)
Appearance and packaging of the product
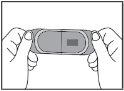
Ditralia 25,000 IU is a rectangular, flexible, light orange film (15 mm x 30 mm).
The orodispersible film is presented in a pack containing 2 or 4 films for each concentration. Each sachet contains one orodispersible film.
Marketing Authorization Holder:
IBSA Farmaceutici Italia S.r.l.
Via Martiri di Cefalonia 2
26900 Lodi, Italy
Manufacturer:
IBSA Farmaceutici Italia S.r.l
S.S. n. 11 Padana Superiore Km 160
20051 Cassina de’ Pecchi (Mi), Italy
or
Altergon Italia Srl
Zona Industriale
83040 Morra de Sanctis (AV), Italy
You can request more information about this medicine by contacting the local representative of the Marketing Authorization Holder:
Instituto Bioquimico Iberico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (Spain)
Date of last revision of this leaflet:
August 2024
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DITRALIA 25,000 IU ORALLY DISINTEGRATING TABLETSDosage form: CAPSULE, 25,000 IUActive substance: colecalciferolManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: CAPSULE, 50,000 IUActive substance: colecalciferolManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: CAPSULE, 50,000 IUActive substance: colecalciferolManufacturer: Consilient Health LimitedPrescription required
Alternatives to DITRALIA 25,000 IU ORALLY DISINTEGRATING TABLETS in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to DITRALIA 25,000 IU ORALLY DISINTEGRATING TABLETS in Poland
Alternative to DITRALIA 25,000 IU ORALLY DISINTEGRATING TABLETS in Ukraine
Online doctors for DITRALIA 25,000 IU ORALLY DISINTEGRATING TABLETS
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for DITRALIA 25,000 IU ORALLY DISINTEGRATING TABLETS – subject to medical assessment and local rules.