
DESFERIN 500 mg POWDER FOR INJECTABLE SOLUTION OR INFUSION


How to use DESFERIN 500 mg POWDER FOR INJECTABLE SOLUTION OR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PATIENT INFORMATION LEAFLET
Desferin 500 mg powder for solution for injection or infusion
Deferoxamine mesylate
Read all of this leaflet carefully before you start using this medicine.
|
Contents of the pack:
- What is Desferin and what is it used for
- What you need to know before you use Desferin
- How to use Desferin
- Possible side effects
- Storing Desferin
Storage of packaging and additional information
1. What is Desferin and what is it used for
Desferin belongs to a group of medicines called iron chelators. It is used to remove excess iron and aluminum from the blood. This may be necessary in patients with certain types of anemia who frequently require blood transfusions (which can lead to excess iron) and in patients with severe kidney failure undergoing maintenance dialysis (which can lead to excess aluminum).
Desferin is indicated for the treatment of:
- chronic iron overload, for example due to frequent blood transfusions in the case of beta-thalassemia major
- acute iron poisoning
- chronic aluminum overload in patients with kidney failure undergoing maintenance dialysis
Desferin may be used to detect iron or aluminum overload.
2. What you need to know before you use Desferin
Desferin is indicated for some patients, but not for all.
Do not use Desferin
- If you are allergic (have ever had a skin rash or difficulty breathing) after using Desferin in the past, you should inform your doctor.
Warnings and precautions
- Desferin should only be administered by slow intramuscular or subcutaneous injection or by slow intravenous infusion. DO NOT USE rapid intravenous infusion as it may lead to shock.
- If you have severe kidney disease.
- Never use a higher dose or concentration of Desferin than that indicated by your doctor, as this may cause side effects at the injection site, or even affect your vision or hearing, lungs, nervous system, or growth rate.
- If your doctor prescribes vitamin C supplements, make sure you have been taking Desferin regularly for at least 1 month and only take the vitamin C doses prescribed by your doctor. High doses of vitamin C (over 500 mg daily) given during treatment with Desferin for chronic iron overload may harm your heart.
- Desferin may increase the risk of certain serious infections. If you experience fever, sore throat, difficulty breathing, abdominal pain, or general discomfort during treatment with Desferin, these could be symptoms of a serious infection. In these cases, stop treatment with Desferin and inform your doctor immediately.
- During treatment, it is possible that your urine may turn a reddish-brown color due to the increased iron content. This is usually harmless, but if you are concerned, talk to your doctor or nurse.
Your doctor may perform blood or urine tests, and may conduct vision and hearing tests before using this medicine and at regular intervals thereafter. In children, growth and body weight will be monitored periodically.
Using other medicines
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those obtained without a prescription, especially if you are taking a tranquilizer called prochlorperazine. You may need to change the dose or stop taking one of the medicines.
Also, do not take vitamin C in doses over 200 mg daily during treatment with Desferin.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medicine.
In general, Desferin should not be taken during pregnancy or breastfeeding, unless your doctor advises treatment. Desferin may harm the child. Your doctor will advise you.
Driving and using machines
Do not drive or operate tools or machines because Desferin may affect your vision or hearing, make you feel dizzy, or cause other nervous system disorders.
Use in children
Desferin may be administered to children. In children under 3 years of age, growth should be monitored.
Use in elderly
Desferin may be administered to elderly patients. The minimum effective dose will be used.
3. How to use Desferin
Follow your doctor's instructions for administering Desferin exactly. Consult your doctor if you have any doubts.
Your doctor will choose the dose and method of administration suitable for your specific situation and will indicate the duration of your treatment with Desferin.
Make sure to take the medication regularly and exactly as your doctor has indicated. This will help you get the best results and reduce the risk of side effects. If you have doubts about your treatment, consult your doctor.
Desferin should be reconstituted with water for injectable preparations. At the recommended concentration of 10%, the reconstituted solution is colorless to yellowish. The solution must be clear. Do not use opaque or cloudy solutions.
The reconstituted Desferin solution may be further diluted with commonly used infusion solutions (NaCl 0.9%, glucose 5%, Ringer's solution, Ringer-Lactate solution, peritoneal dialysis solution such as Dianeal 137 Glucose 2.27%, Dianeal PD4 Glucose 2.27%, and CAPD/DPCA 2 Glucose 1.5%).
Treatment of chronic iron overload
Your doctor will adjust the dose according to your specific situation. In most patients, a daily dose of 20-60 mg per kilogram of body weight is suitable.
Desferin may be administered by slow subcutaneous infusion with an infusion pump, by intravenous infusion, or by intramuscular injection.
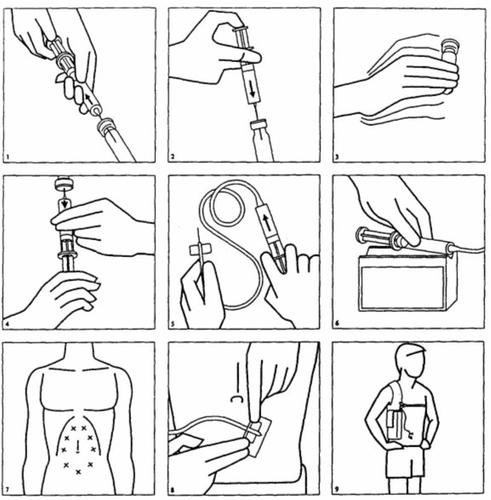
In long-term treatments in patients with iron overload, it is convenient to administer Desferin slowly by subcutaneous infusion using a portable, lightweight pump over a period of 8-12 hours (e.g., during the night). The pump should be installed carefully under aseptic conditions. Follow these instructions to prepare the infusion solution and to inject it subcutaneously:
- Aspirate the necessary water for injectable preparations into a syringe:
- for subcutaneous use (infusion pump) or intravenous use: 5 ml of water for injectable preparations (concentration after reconstitution: 95 mg/ml)
- for intramuscular use: 2 ml of water for injectable preparations (concentration after reconstitution: 213 mg/ml)
- After cleaning the rubber stopper of the Desferin vial with alcohol, inject the contents of the syringe into the vial.
- Vigorously shake the vial to dissolve the powder.
- Aspirate the dissolved medication with the syringe.
- Attach the infusion tube to the syringe, connect the infusion tube to a butterfly needle, and then fill the empty space in the tube with the injectable solution.
- Then, place the syringe in the infusion pump.
- For infusion, the butterfly needle can be inserted under the skin of the abdomen, arm, upper leg, or thigh. It is essential to clean the skin well with alcohol first. Then, firmly insert the needle, up to the wings, into a skin fold formed by the free hand. The tip of the needle should move freely when the needle is moved. If it does not move freely, the tip of the needle is too close to the skin. Try again at a new site after cleaning with alcohol.
- Secure the needle well by taping it.
- Usually, the pump is carried on the body, attached to a belt or straps. Nighttime use is most suitable for the patient.
It is possible that your doctor may decide that you should take vitamin C in addition to Desferin, starting at least one month after regular treatment with Desferin. The maximum daily dose of vitamin C for adults is 200 mg, divided into several doses. For children under 10 years of age, 50 mg of vitamin C per day is sufficient, and for children over 10 years of age, 100 mg is sufficient.
Treatment of acute iron poisoning
Desferin may be used in cases of iron poisoning. This treatment will be performed in a hospital. In this case, the medication will be administered by continuous intravenous infusion.
Treatment of chronic aluminum overload
Desferin is usually administered once a week by slow intravenous infusion during the last 60 minutes of a dialysis session or 5 hours before the dialysis session, depending on the aluminum concentration in the blood.
If you are receiving continuous ambulatory peritoneal dialysis (CAPD) or continuous cyclic peritoneal dialysis (CCPD), you will need to use the Desferin dose before the final exchange of the day.
The dose of Desferin is 5 mg per kilogram of body weight.
The duration of treatment and any change in the individual dose of Desferin will depend on the results of the tests performed by your doctor.
Desferin test
If your doctor wants to check if you have an iron overload, they will administer 500 mg of Desferin into the muscle. The iron content will be determined in the urine collected over 6 hours.
If you are undergoing dialysis, your doctor will want to check if you have an aluminum overload. You will be administered 5 mg of Desferin per kilogram of body weight by slow intravenous infusion during the last 60 minutes of the dialysis session. The aluminum content will be determined in blood samples taken just before this dialysis session and the next one.
Use in children
If chelation therapy is started before the age of 3, growth should be closely monitored, and the average daily dose should not exceed 40 mg/kg. Growth delay could be a consequence of iron overload or excessive doses of Desferin.
Patient over 65 years
In general, the determination of the dose in elderly patients should be done with caution, usually starting with the lower dose range, because in elderly patients, it is more common for the liver, kidney, or heart not to function properly, and there may be associated diseases and the use of other medications.
If you use more Desferin than you should
Inform your doctor immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicology Information Service. Phone 915 620 420, indicating the medication and the amount used.
If you forget to use Desferin
Inform your doctor immediately.
4. Possible side effects
Like all medicines, Desferin can cause side effects, although not everybody gets them.
A rapid intravenous infusion of Desferin can cause unpleasant side effects, even leading to shock.
Very common (may affect more than 1 in 10 people)
Musculoskeletal and connective tissue disorders: Joint pain, muscle pain. | |
General disorders and administration site conditions: Reaction at the injection site, including pain, swelling, redness, itching, and wound. |
Common (may affect up to 1 in 10 people)
Nervous system disorders: Headache. | |
Gastrointestinal disorders: Nausea. | |
Skin and subcutaneous tissue disorders: Urticaria. | |
Musculoskeletal and connective tissue disorders: Growth delay and bone disorders in high doses and young children. | |
General disorders and administration site conditions: Fever. |
Uncommon (may affect up to 1 in 100 people)
Ear and labyrinth disorders: Hearing loss and ringing in the ears. | |
Respiratory, thoracic, and mediastinal disorders: Asthma. | |
Gastrointestinal disorders: Vomiting, abdominal pain. | |
General disorders and administration site conditions: Reaction at the injection site, including blisters, inflammation, and burning. |
Rare (may affect up to 1 in 1,000 people)
Infections and infestations: Fungal infections. | |
Eye disorders: Vision loss, black spots, decreased visual acuity, blurred vision, night blindness, visual field defects, color vision disturbances, retinal degeneration, cataracts. | |
Vascular disorders: Hypotension, tachycardia, and shock if precautions for administration are not followed. |
Very rare (may affect up to 1 in 10,000 people)
Infections and infestations: Fungal or bacterial infections accompanied by fever, diarrhea, abdominal pain, or sore throat and painful inflammation (sign of low white blood cell count). | |
Blood and lymphatic system disorders: Bleeding and bruising (sign of low platelet count); fever, sore throat, or mouth sores due to infections (sign of low white blood cell count). | |
Immune system disorders: Severe allergic reaction, with difficulty breathing and dizziness. | |
Nervous system disorders: Neurological disorders, including dizziness, worsening of dialysis-related aluminum encephalopathy (personality changes, severe headache, confusion, partial paralysis, torticollis, abnormal eye movements, and abnormal speech), numbness, and tingling in feet and hands. | |
Respiratory, thoracic, and mediastinal disorders: Severe breathing difficulties. | |
Gastrointestinal disorders: Diarrhea. | |
Skin and subcutaneous tissue disorders: Widespread rash. | |
Frequency not known (cannot be estimated from the available data)
Renal and urinary disorders: Low urine production (sign of kidney problem); abnormal kidney function test results, urine discoloration. | |
Nervous system disorders: Seizure (mainly in patients on dialysis). | |
Musculoskeletal and connective tissue disorders: Muscle spasms. | |
Gastrointestinal disorders: Abnormal liver function test results. Endocrine disorders: In treatment for aluminum overload, it may result in low calcium levels and worsening of hyperparathyroidism (a disease with abnormally high functioning of the parathyroid glands that regulate calcium, phosphorus, and vitamin D levels in the blood/bones). |
If you think you have experienced any of the side effects mentioned, or if you notice any side effects not mentioned in this leaflet, inform your doctor or pharmacist.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not mentioned in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Desferin
Keep out of the reach and sight of children.
Do not use Desferin after the expiration date stated on the packaging (the expiration date is the last day of the month indicated).
Do not use Desferin if you notice the solution is opaque or cloudy.
Do not store above 30°C.
The vials are for single use. Once the solution is reconstituted, the product must be used immediately (within 3 hours). If the solution is reconstituted under sterile conditions, it can be stored for a maximum of 24 hours at room temperature before the start of treatment.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medication at the SIGRE collection point in your usual pharmacy. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Additional Information
Composition of Desferin
The active ingredient of Desferin is deferoxamine mesylate.
Appearance of the product and packaging contents
Desferin is presented in vials containing 500 mg of white powder for injectable and perfusion solution.
Only clear, colorless to yellowish solutions should be used.
Marketing authorization holder
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona / Spain
Manufacturer responsible
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona - Spain
or
Novartis Pharma GmbH
Roonstrasse 25
90429 Nürnberg - Germany
This leaflet was approved in June 2018
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price36.86 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DESFERIN 500 mg POWDER FOR INJECTABLE SOLUTION OR INFUSIONDosage form: TABLET, 180 mgActive substance: deferasiroxManufacturer: Abdi Farma GmbhPrescription requiredDosage form: TABLET, 360 mgActive substance: deferasiroxManufacturer: Abdi Farma GmbhPrescription requiredDosage form: TABLET, 90 mgActive substance: deferasiroxManufacturer: Abdi Farma GmbhPrescription required
Online doctors for DESFERIN 500 mg POWDER FOR INJECTABLE SOLUTION OR INFUSION
Discuss questions about DESFERIN 500 mg POWDER FOR INJECTABLE SOLUTION OR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






