DELTIUS 10,000 IU/mL ORAL DROPS IN SOLUTION


How to use DELTIUS 10,000 IU/mL ORAL DROPS IN SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Deltius 10,000 IU/ml Oral Drops in Solution
colecalciferol (vitamin D3)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Deltius and what is it used for
- What you need to know before you take Deltius
- How to take Deltius
- Possible side effects
- Storage of Deltius
- Contents of the pack and other information
1. What is Deltius and what is it used for
Deltius oral drops in solution contain colecalciferol (vitamin D3). Vitamin D3 can be found in some foods and is produced by the body when the skin is exposed to sunlight. Vitamin D helps the body absorb calcium in the kidneys and intestines, which helps form bones. A deficiency of vitamin D is the main cause of rickets (poor bone mineralization in children) and osteomalacia (inadequate bone mineralization in adults).
Deltius oral drops in solution are used to prevent and treat vitamin D3 deficiency in adults, adolescents, and children with an identified risk of vitamin D3 deficiency.
Deltius oral drops in solution can also be used as an adjunct to specific medication for bone loss.
2. What you need to know before you take Deltius
Do not take Deltius
- if you are allergic to vitamin D3 or any of the other ingredients of this medicine (listed in section 6).
- if you have high levels of calcium in your blood (hypercalcemia) or urine (hypercalciuria).
- if you have kidney stones (renal calculi) or severe kidney failure.
- if you have high levels of vitamin D in your blood (hypervitaminosis D).
Warnings and precautions
Consult your doctor or pharmacist before starting to take Deltius if:
- you are being treated with certain medicines for heart conditions (e.g., cardiac glycosides, such as digoxin).
- you have sarcoidosis (an autoimmune disease that can cause increased levels of vitamin D in the body).
- you are being treated with medicines that contain vitamin D or taking foods or milk enriched with vitamin D.
- you are likely to be exposed to the sun while using this medicine.
- you are taking other supplements that contain calcium. Your doctor should monitor your calcium levels to ensure they are not too high during your treatment with Deltius.
- you have kidney disease. Your doctor should monitor your blood and urine calcium levels.
- your doctor should monitor your blood calcium levels through laboratory tests if your daily intake of vitamin D3 exceeds 1,000 IU for a prolonged period.
Other medicines and Deltius
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This is especially important if you are taking:
- medicines used to treat heart or kidney diseases, such as cardiac glycosides (e.g., digoxin) or diuretics (e.g., bendroflumethiazide). When these medicines are used at the same time as vitamin D3, they can cause a significant increase in blood and urine calcium levels.
- medicines that contain vitamin D or foods rich in vitamin D, such as some types of vitamin D-enriched milk.
- actinomycin (a medicine used to treat certain types of cancer) and imidazole antifungals (e.g., clotrimazole and ketoconazole, which are medicines used to treat fungal diseases). These medicines can interfere with the processing of vitamin D3 in the body.
- the following medicines, as they may interfere with the effect or absorption of vitamin D3:
- antiepileptic medicines (anticonvulsants), barbiturics.
- glucocorticoids (steroid hormones such as hydrocortisone or prednisolone), as they may reduce the effect of vitamin D3.
- medicines that lower blood cholesterol levels (such as cholestyramine or colestipol).
- certain medicines used to lose weight by reducing fat absorption (e.g., orlistat).
- certain laxatives (such as liquid paraffin).
Using Deltius with food, drinks, and alcohol
You should take Deltius preferably with one of the main meals to help with the absorption of vitamin D3. You can take it alone or mix the drops with cold or lukewarm food. For more information, see section 3 "How to take Deltius".
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. Deltius should be taken during pregnancy and breastfeeding only under the recommendation of your doctor.
Overdoses of vitamin D should be avoided during pregnancy, as prolonged hypercalcemia can cause physical and mental developmental delays, supravalvular aortic stenosis, and retinopathy in the child.
Driving and using machines
Information on the possible effects of this medicine on the ability to drive is limited. However, it is not expected to affect the ability to drive and use machines.
3. How to take Deltius
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Shake before use.
Deltius should be taken preferably with the main meals.
This medicine has an olive oil flavor. Deltius can be taken alone or by mixing the prescribed number of drops with a spoonful or a small amount of cold or lukewarm food immediately before ingestion. You must ensure that you take the complete dose.
Use in adults
The recommended dose for vitamin D deficiencyand as a complement to specific medication for bone loss (osteoporosis) is 3-4 drops (600 IU - 800 IU) per day.
For the treatment of vitamin D deficiency, the dose is usually 4 drops (800 IU) per day. Higher doses should be adjusted depending on the desired serum levels of 25-hydroxycolecalciferol (25(OH)D), the severity of the disease, and the patient's response to treatment.
The daily dose should not exceed 4,000 IU (20 drops per day).
Use in children and adolescents
For the prevention of vitamin D deficiency in children (from 0 to 11 years) with an identified risk, the recommended dose is 2 drops (400 IU) per day. For prevention in adolescents (from 12 to 18 years) with an identified risk, the recommended dose is 3-4 drops (600-800 IU) per day.
For the treatment of vitamin D deficiency in children (from 0 to 11 years) and adolescents (from 12 to 18 years), the dose should be adjusted depending on the desired serum levels of 25-hydroxycolecalciferol (25(OH)D), the severity of the disease, and the patient's response to treatment. The daily dose should not exceed 1,000 IU per day for children under 1 year, 2,000 IU per day for children from 1 to 10 years, and 4,000 IU per day for adolescents.
In children, Deltius can be mixed with a small amount of baby food, yogurt, milk, cheese, or other dairy products. Do not add Deltius to baby bottles or other containers with food that the child will not consume completely, to avoid the child not taking the complete dose. You must ensure that the child takes the complete dose. In the case of children who have passed the breastfeeding stage, the prescribed dose should be administered with some main meal.
Do not store any product or food containing Deltius for later use or in the next meal.
Use during pregnancy and breastfeeding
The recommended dose is 400 IU/day (2 drops). Higher doses may be necessary after confirmation of vitamin D deficiency, but do not take more than your doctor recommends.
Instructions for usefor the bottle withdropper
The package contains a bottle and a dropper. The bottle is closed with a child-resistant plastic cap. The dropper is protected by a plastic tube. To use the medicine, shake the bottle before use and follow the instructions below:
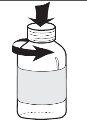
- To open the bottle, press the cap down and turn it at the same time (see Figure 1). Do not throw away the plastic cap, you will use it to close it;
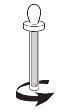
- Before using the dropper, unscrew the plastic tube that protects it (see Figure 2). Do not throw away the plastic tube, you will use it to store it after each use;
- Insert the glass rod of the dropper into the bottle to extract the contents. Put the prescribed number of drops in a spoon;
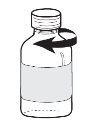
- Close the bottle with its original cap and store the dropper in the plastic tube. Make sure both are well closed (see Figure 3);
- Store the bottle and dropper in the original package.
Figure 1 |
Figure 2 |
Figure 3 |
Instructions for use of the dropper bottle
The box contains a dropper bottle. The dropper bottle is closed with a child-resistant cap.
The following are the instructions for opening and use:
- To open the dropper bottle, press and turn the plastic cap at the same time;
- Put the dropper bottle upside down in a vertical position and collect the prescribed number of drops in a spoon;
- After finishing the administration of the drops, put the dropper bottle back in a vertical position upwards;
- To close the dropper bottle, screw the child-resistant plastic cap back on
- Place the dropper bottle in the original carton package.
If you take more Deltius than you should
Stop taking the medicine and consult your doctor or pharmacist immediately if you or your child take more of this medicine than prescribed by your doctor. If it is not possible to talk to your doctor, go to the nearest hospital and take the medicine package with you.
You can also contact the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount taken.
The most frequent symptoms in case of overdose are: nausea, vomiting, excessive thirst, excessive urine production for about 24 hours, constipation, and dehydration, and high levels of calcium in the blood and urine (hypercalcemia and hypercalciuria) in clinical tests.
If you forget to take Deltius
If you forget to take a dose of Deltius, take the missed dose as soon as possible. Then, take the next dose at the usual time. However, if it is close to the time of the next dose, do not take the missed dose, and take the next dose at the usual time.
Do not take a double dose to make up for missed doses.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The possible side effects associated with the use of Deltius may be:
Uncommon(affect less than 1 in 100 people):
- Excess calcium in the blood (hypercalcemia)
- Excess calcium in the urine (hypercalciuria).
Rare(affect less than 1 in 1,000 people):
- Skin rash (rash or skin eruption)
- Itching (pruritus)
- Urticaria.
Reporting of side effects:
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es/. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Deltius
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the package, after EXP. The expiry date is the last day of the month shown.
Do not store above 30°C.
Store in the original package to protect from light.
Do not refrigerate or freeze.
After the first opening of the package, the medicine can be used for a maximum period of 6 months.
Do not use this medicine if you notice that the solution is cloudy.
Medicines should not be disposed of via wastewater or household waste. Place the package and any unused medicine in the SIGRE collection point at the pharmacy. If you have any further questions, ask your pharmacist how to dispose of the package and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition ofDeltius
The active ingredient is colecalciferol (vitamin D3).
1 ml of oral drops in solution (50 drops) contains 10,000 IU of colecalciferol (vitamin D3), equivalent to 0.25 mg.
1 drop contains 200 IU of colecalciferol (vitamin D3).
The other ingredients are: olive oil.
Appearance of Deltius and contents of the pack
Deltius 10,000 IU/ml oral drops in solution is a clear, colorless to yellow-green oral oil solution, without visible solid particles and/or precipitates. It is presented in:
- Bottle with dropper
Brown glass bottle of 20 ml closed with a child-resistant plastic cap.
Each package contains a brown glass bottle containing 10 ml of solution (corresponding to 500 drops) and a dropper (with CE 0068 marking).
- Dropper bottle
Brown glass dropper bottle of 20 ml with a child-resistant plastic cap.
Each package contains a brown glass dropper bottle containing 10 ml of solution (corresponding to 500 drops).
Not all presentations may be marketed.
Marketing authorization holder
ITALFARMACO, S.A.
San Rafael, 3
28108 Alcobendas, Madrid
Spain
Manufacturer
Abiogen Pharma S.p.A.
Via Meucci, 36
Pisa
Italy
This medicine is authorized in the Member States of the European Economic Area under the following names:
Spain: Deltius 10,000 IU/ml oral drops in solution
UK: Deltius 10,000 I.U./ml oral drops, solution
Greece: Deltius Π?σιμες σταγ?νες δι?λυμα 10,000 IU/ML
France: Deltius 10,000 UI/ml, solution buvable en gouttes
Portugal: Deltius 10,000 UI/ml Gotas orais, solução
Date of the last revision of this leaflet:April 2021
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price9.37 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DELTIUS 10,000 IU/mL ORAL DROPS IN SOLUTIONDosage form: CAPSULE, 25,000 IUActive substance: colecalciferolManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: CAPSULE, 50,000 IUActive substance: colecalciferolManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: CAPSULE, 50,000 IUActive substance: colecalciferolManufacturer: Consilient Health LimitedPrescription required
Online doctors for DELTIUS 10,000 IU/mL ORAL DROPS IN SOLUTION
Discuss questions about DELTIUS 10,000 IU/mL ORAL DROPS IN SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions