
CYANOKIT 5 g POWDER FOR SOLUTION FOR INFUSION

How to use CYANOKIT 5 g POWDER FOR SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cyanokit 5 g powder for solution for infusion
Hydroxocobalamin
Read all of this leaflet carefully before using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack:
- What is Cyanokit and what is it used for
- What you need to know before taking Cyanokit
- How to take Cyanokit
- Possible side effects
- Storage of Cyanokit
- Contents of the pack and further information
1. What is Cyanokit and what is it used for
Cyanokit contains the active substance hydroxocobalamin.
Cyanokit is an antidote for the treatment of confirmed or suspected cyanide poisoning in all age groups.
Cyanokit should be administered together with appropriate decontamination and supportive therapy measures.
Cyanide is a highly toxic chemical substance. Cyanide poisoning can be caused by exposure to smoke from domestic or industrial fires, inhalation or ingestion of cyanide, or by skin contact with cyanide.
2. What you need to know before taking Cyanokit
Warnings and precautions
Tell your doctor or other healthcare professional:
- If you are allergic to hydroxocobalamin or vitamin B12. They will take this into account before treating you with Cyanokit.
- If you have received treatment with Cyanokit if you need to undergo any of the following procedures:
- any blood or urine test. Cyanokit may alter the results of these tests.
- a burn assessment. Cyanokit may interfere with the assessment due to the red coloration of the skin.
- hemodialysis. Cyanokit may cause the dialysis machines to shut down until it has been eliminated from the blood (at least 5.5 to 6.5 days).
- renal function monitoring: Cyanokit may lead to renal failure and the presence of crystals in the urine.
Using Cyanokit with other medicines
Tell your doctor or other healthcare professional if you are taking, have recently taken, or might take any other medicine.
Detailed information for your doctor or other healthcare professional on the simultaneous administration of Cyanokit with other medicines can be found at the end of this leaflet (see "Instructions for use and handling").
Pregnancy and breastfeeding
This medicine is an emergency treatment. It can be administered during pregnancy and breastfeeding.
Tell your doctor as soon as possible if you were pregnant or think you may have been pregnant during treatment with Cyanokit.
Your doctor will advise you to stop breastfeeding after treatment with Cyanokit.
3. How to take Cyanokit
Your doctor or healthcare professional will administer Cyanokit to you by infusion into a vein. You may need one or two infusions.
The first infusion of Cyanokit that you will be given will last 15 minutes. In adults, the initial dose is 5 g. In children, it is 70 mg/kg body weight, up to a maximum dose of 5 g. If you need a second infusion, it will last between 15 minutes and 2 hours, depending on the severity of your poisoning. The maximum recommended total dose is 10 g in adults and 140 mg/kg, up to a maximum of 10 g, in children.
Detailed instructions for your doctor or other healthcare professional on how to prepare the Cyanokit infusion and determine the dose can be found at the end of this leaflet (see "Instructions for use and handling").
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Cyanokit can cause side effects, although not everybody gets them. The following side effects may occur (frequency cannot be estimated from the available data):
Allergic reaction (hypersensitivity)
Tell your doctor immediatelyif you experience any of the following symptoms during or after treatment:
- Swelling around the eyes, lips, tongue, throat, or hands.
- Difficulty breathing, hoarseness, or difficulty speaking.
- Redness of the skin, skin rash with irritation (urticaria) or itching.
These side effects can be severe and may require immediate attention.
Cardiac and blood pressure problems
- Symptoms such as headache or dizziness, as they may be due to an increase in blood pressure. This increase in blood pressure occurs especially at the end of the administration of this treatment and usually resolves after a few hours.
- Irregular heartbeat.
- Redness of the face (flushing).
In patients who suffer from cyanide poisoning, a decrease in blood pressure and an increase in heart rate have also been observed.
Respiratory and thoracic problems
- Presence of fluid in the chest (pleural effusion)
- Difficulty breathing
- Sensation of pressure in the throat
- Dryness in the throat
- Chest pressure (in the chest).
Kidney and urinary problems
- Kidney damage such as acute renal failure and crystals in the urine.
- Red coloration of the urine.
All patients will show a dark red coloration of the urine, quite marked during the first three days after administration. The coloration of the urine may persist for up to 35 days after administration of Cyanokit. This coloration has no other consequences for the body.
Gastrointestinal problems (digestive)
- Stomach discomfort
- Indigestion
- Diarrhea
- Nausea
- Vomiting
- Difficulty swallowing.
Eye problems
- Inflammation, irritation, redness. Skin reactions
- Most patients will experience reversible red coloration of the skin and mucous membranes that can persist for up to 15 days after administration of Cyanokit.
- Pustular eruptions on the skin. These can persist for several weeks and mainly affect the face and neck.
- Inflammation of the part of the body where the medicine was administered by infusion.
Other side effects
- Restlessness
- Memory problems
- Dizziness
- Headache
- Swelling of the ankles
- Changes in the results of some blood tests related to blood cells (lymphocytes)
- Coloration of the plasma, which can cause an artificial increase or decrease in some laboratory values.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cyanokit
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial, carton, and blister after "EXP".
Do not store above 25°C.
For ambulatory use, Cyanokit can be exposed to temperature variations for:
- usual transport (15 days at temperatures between 5°C and 40°C)
- desert transport (4 days at temperatures between 5°C and 60°C) and
- freeze-thaw cycles (15 days at temperatures between -20°C and 40°C).
For the storage conditions of the reconstituted medicine, see "Instructions for use and handling" at the end of this leaflet.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Cyanokit
- The active substance is hydroxocobalamin. The vial contains 5 g of hydroxocobalamin. After reconstitution with 200 mL of solvent, each mL of the reconstituted solution contains 25 mg of hydroxocobalamin.
- The other component is hydrochloric acid (for pH adjustment).
Appearance and pack contents
Cyanokit powder for solution for infusion is a dark red crystalline powder, supplied in a glass vial closed with a bromobutyl rubber stopper and an aluminum seal with a plastic cap.
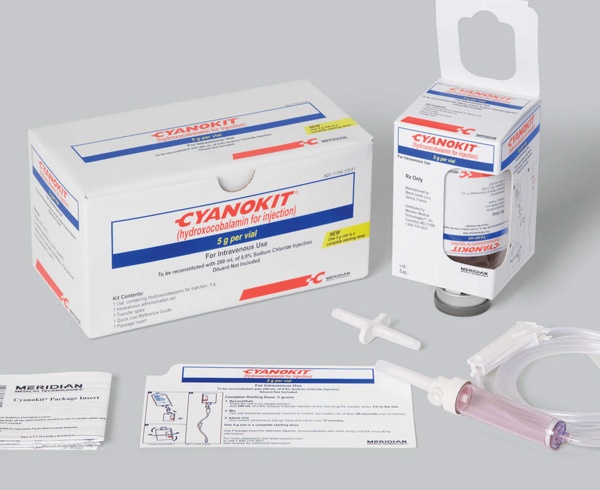
Each pack contains a vial packaged in a carton, a sterile transfer device, a sterile intravenous infusion set, and a short sterile catheter for administration to children.
Marketing authorisation holder
SERB S.A.
Avenue Louise 480
1050 Brussels
Belgium
Manufacturer
Merck Santé s.a.s. / SEMOY
2, rue du Pressoir Vert
45400 Semoy
France
Or
SERB S.A.
Avenue Louise 480
1050 Brussels
Belgium
Or
SERB
40 avenue George V
75008 Paris
France
Date of last revision of this leaflet: MM/AAAA.
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
---------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Instructions for use and handling
Treatment of cyanide poisoning should include immediate medical attention, ensuring airway patency, adequate oxygenation and hydration, cardiovascular care, and treatment of seizures. Decontamination measures should be considered depending on the route of exposure.
Cyanokit does not replace oxygen therapy and should not delay the establishment of the above measures. Frequently, the presence and magnitude of cyanide poisoning are not initially known. There is no widely available and rapid test to confirm the presence of cyanide in the blood. However, if determination of the cyanide concentration in the blood is planned, it is recommended to draw the blood sample before starting treatment with Cyanokit. Therapeutic decisions should be based on clinical history and/or the presence of signs and symptoms of cyanide poisoning. If there is a clinical suspicion of cyanide poisoning, it is strongly recommended to administer Cyanokit without delay.
Preparation of Cyanokit
The vial should be reconstituted with 200 mL of solvent, using the sterile transfer device provided. The recommended solvent is sodium chloride 9 mg/mL (0.9%) solution for injection. Only if sodium chloride 9 mg/mL (0.9%) solution for injection is not available, can Ringer's lactate solution or glucose 50 mg/mL (5%) solution for injection also be used.
The Cyanokit vial should be swirled or inverted for at least one minute to mix the solution. It should not be shaken, as this may lead to foam formation and make it difficult to check the reconstitution. Because the reconstituted solution is a dark red solution, some insoluble particles may not be visible. For this reason, the provided intravenous infusion set should be used, which contains a suitable filter that should be primed with the reconstituted solution.
Dosage
Initial dose
Adults:The initial dose of Cyanokit is 5 g (200 mL, full volume of the reconstituted solution).
Pediatric population:From infants to adolescents (0 to 18 years), the initial dose of Cyanokit is 70 mg/kg body weight, not exceeding 5 g.
Body weight | |||||||
in kg | 5 | 10 | 20 | 30 | 40 | 50 | 60 |
Initial dose | |||||||
in g | 0.35 | 0.70 | 1.40 | 2.10 | 2.80 | 3.50 | 4.20 |
in mL | 14 | 28 | 56 | 84 | 112 | 140 | 168 |
Subsequent dose
Depending on the severity of the poisoning and the clinical response, a second dose may be administered.
Adults:The subsequent dose of Cyanokit is 5 g (200 mL, full volume of the reconstituted solution).
Pediatric population:From infants to adolescents (0 to 18 years), the subsequent dose of Cyanokit is 70 mg/kg body weight, not exceeding 5 g.
Maximum dose
Adults:The maximum recommended total dose is 10 g.
Pediatric population:From infants to adolescents (0 to 18 years), the maximum recommended total dose is 140 mg/kg, not exceeding 10 g.
Renal or hepatic impairment
In these patients, no dose adjustment is required.
Method of administration
The initial dose of Cyanokit is administered by intravenous infusion over 15 minutes.
The rate of the intravenous infusion for the second dose ranges from 15 minutes (for highly unstable patients) to 2 hours, depending on the patient's condition.
Simultaneous administration of Cyanokit and other products
Cyanokit should not be mixed with other diluents, except with sodium chloride 9 mg/mL (0.9%) solution, Ringer's lactate solution, or glucose 50 mg/mL (5%) solution for injection.
As physical and chemical incompatibilities have been observed with several commonly used resuscitation medications, these or any other medications should not be administered simultaneously in the same intravenous line as hydroxocobalamin.
If blood products (blood, red blood cell concentrate, platelet concentrate, and fresh frozen plasma) and hydroxocobalamin are to be administered simultaneously, it is recommended to use separate intravenous lines (preferably in contralateral limbs).
Combination with another cyanide antidote:chemical incompatibility was observed with sodium thiosulfate and sodium nitrite. If it is decided to administer another cyanide antidote with Cyanokit, these medications should not be administered at the same time in the same intravenous line.
Stability during use of the reconstituted solution
The chemical and physical stability of the reconstituted solution with sodium chloride 9 mg/mL (0.9%) has been demonstrated for 6 hours, at a temperature between 2°C and 40°C.
From a microbiological point of view, the medicine should be used immediately. If not used immediately, the storage time and conditions prior to use are the responsibility of the user and normally should not exceed 6 hours, at a temperature of 2°C to 8°C.
- Country of registration
- Average pharmacy price812.5 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CYANOKIT 5 g POWDER FOR SOLUTION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 2.5 gActive substance: hydroxocobalaminManufacturer: Serb SaPrescription requiredDosage form: INJECTABLE, 0.5 mg/5 mlActive substance: flumazenilManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: INJECTABLE, 1 mg flumazenil/10 mlActive substance: flumazenilManufacturer: Cheplapharm Arzneimittel GmbhPrescription required
Frequently Asked Questions





