
Colobreathe 1,662,500 IU powder for inhalation, hard capsules

How to use Colobreathe 1,662,500 IU powder for inhalation, hard capsules
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Colobreathe 1.662.500 UI powder for inhalation, hard capsules
colistimethate sodium
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Colobreathe and what is it used for
- What you need to know before you use Colobreathe
- How to use Colobreathe
- Possible side effects
- Storing Colobreathe
- Contents of the pack and other information
1. What is Colobreathe and what is it used for
Colobreathe contains colistimethate sodium, a type of antibiotic called polymyxin.
Colobreathe is used to control persistent lung infections caused by the bacterium Pseudomonas aeruginosain adult and child patients 6 years of age and older with cystic fibrosis. Pseudomonas aeruginosais a very common bacterium that infects almost all patients with cystic fibrosis at some point in their lives. Some people get this infection while they are very young, but for others it happens much later. If this infection is not properly controlled, it will cause lung damage.
How it works
Colobreathe works by destroying the bacterial cell membrane, with a lethal effect on these bacteria.
2. What you need to know before you use Colobreathe
Do not use Colobreathe:
- if you/your child is allergic to colistimethate sodium, colistin sulfate or polymyxins.
Warnings and precautions
Talk to your doctor or pharmacist before you start using Colobreathe.
Tell your doctor if you/your child has ever suffered from any of the following conditions:
- has previously reacted badly to the inhalation of dry powder medicines, unless this has already been discussed with your doctor.
- already has a known muscle disease called myasthenia gravis or the hereditary disease porphyria
- blood in the sputum (the matter you cough up)
After each inhalation of Colobreathe, you should rinse your mouth with water. The rinse should not be swallowed. The rinse can reduce the risk of developing oral fungal infections during treatment and can also reduce the unpleasant taste associated with colistimethate sodium.
When you/your child starts using Colobreathe, you/your child may find that you have a cough, difficulty breathing, chest tightness or wheezing (a whistling sound that occurs when breathing). The number of these side effects can be reduced as you continue to use the inhaler or your doctor may prescribe a bronchodilator for you to use before or after taking Colobreathe. If any of these effects become a problem, please contact your doctor who may change your treatment.
If you/your child has any kidney or nerve problems, you should be extremely careful when taking Colobreathe, although your doctor will already be aware of this.
If you/your child needs to take other forms of colistimethate, either by injection or by nebulisation, you should be extremely careful, although your doctor will already be aware of this.
Children
Do not give Colobreathe to children under 6 years of age, as it is not suitable for them.
Other medicines and Colobreathe
Tell your doctor if you/your child is taking, has recently taken or might take any other medicines, and in particular:
- if you/your child is taking aminoglycoside antibiotics used to treat infections, you should be extremely careful;
- if you/your child has myasthenia gravis and is taking macrolide antibiotics such as azithromycin and clarithromycin, or fluoroquinolones such as norfloxacin and ciprofloxacin. Taking these at the same time as Colobreathe may cause muscle weakness problems;
- if you/your child is taking colistimethate by injection or nebulisation, you should be extremely careful;
- if you/your child needs a general anaesthetic, you should be extremely careful.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
There is no information on the safety of Colobreathe in pregnant women. Your doctor will advise you on whether the benefits of the medicine outweigh the risks.
Colistimethate sodium may pass into breast milk. Discuss the use of Colobreathe with your doctor.
Driving and using machines
You may experience dizziness, confusion or have problems with your vision while using Colobreathe. Do not drive or operate machinery until the symptoms have disappeared.
Colobreathe contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per capsule; this is essentially “sodium-free”.
3. How to use Colobreathe
Follow the instructions for administration of the medicine contained in this leaflet or as directed by your doctor. If you are in doubt, ask your doctor if you/your child has doubts.
The first dose should be given under medical supervision.
The recommended dose is
Adults and children 6 years of age and older
- The contents of one Colobreathe capsule should be inhaled twice a day using the Turbospin inhaler.
- There should be a 12-hour gap between doses.
The order in which you should take or perform other treatments
If you/your child is taking other treatments for cystic fibrosis, you/your child should take them in the following order:
- Inhaled bronchodilators
- Respiratory physiotherapy
- Other inhaled medicines
- Then Colobreathe
You/your child should confirm the order of your treatments with your doctor.
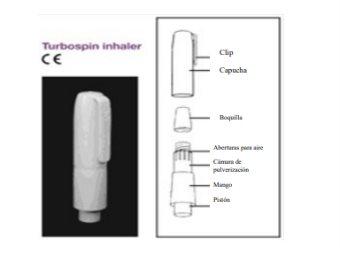
Method of administration
Colobreathe is inhaled into the lungs as a powder from the capsule using the manual inhaler called Turbospin. Colobreathe can only be administered using this device.
Do not swallow Colobreathe capsules.
To inhale Colobreathe from the capsule using the Turbospin inhaler, follow the procedure described below. Your doctor, pharmacist or nurse should show you/your child how to inhale the medicine when you/your child start treatment:
Using Colobreathe with the Turbospin inhaler
Preparing the Turbospin
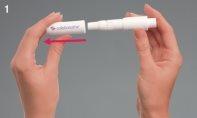
- Remove the hood by gently pulling it off.

- Unscrew the mouthpiece, exposing the Turbospin inhaler chamber.

- Remove one capsule from the blister pack. Once you have removed the capsule, you must use it immediately.

- Gently insert the capsule into the chamber with the wider end first. Do not force it.

- Now replace the mouthpiece by screwing it back into place.
Piercing the capsule and inhaling the medicine

- To pierce the capsule:
- Hold the inhaler with the mouthpiece upwards, gently push the piston upwards until you reach the visible line; you will feel resistance at this point and this will fix the capsule in place, ready to be pierced. Hold this position before proceeding to pierce.
- Now, with the capsule in place, continue to push the piston until you reach the stop and then release it.
- The capsule is now pierced and the contents can be inhaled.
- Do notpierce the capsule more than once. You may see a little powder coming out of the capsule chamber after piercing, which is normal.

- Exhale slowly. Place the mouthpiece between your lips and teeth. Make sure you create a seal between your lips and the mouthpiece. Be careful not to block the air inlets with your fingers or mouth during inhalation.
- Then, inhale slowly and deeply through your mouth at a rate that allows you to hear or feel the capsule spinning.
- Remove the Turbospin inhaler from your mouth and hold your breath for about 10 seconds, or for as long as you feel comfortable, then exhale slowly.
- If you do not hear the capsule spinning, it may be stuck in the compartment. If this happens, you can release it by gently tapping the inhaler chamber. Do not try to release the capsule by repeatedly pressing the piston. If the capsule cannot be released and the powder cannot be inhaled, discard the broken capsule and any remaining powder in it and use another one.
- Inhale the medicine again by repeating steps 7 and 8 to make sure you have emptied the capsule.
- You can check if the capsule is empty by unscrewing the mouthpiece and checking the capsule. If it is not empty, repeat steps 7, 8 and 9 until you have inhaled all the contents.
- When you have inhaled all the contents, rinse your mouth well with water and then spit it out.
Removing the empty capsule from the Turbospin
- When the capsule is empty, unscrew the mouthpiece, then remove and discard the empty capsule.
Additional information
When you breathe in slowly, you draw air through the body of the Turbospin inhaler into the capsule chamber. The tiny particles of medicine from the capsule are picked up by the airflow and carried through your airways to your lungs.
Occasionally, very small pieces of the capsule casing may enter your mouth or airways.
- If this happens, you may notice these pieces on your tongue or in your airways.
- The capsule casing is made of gelatine, which is harmless to humans if swallowed or inhaled.
- The chances of the capsule breaking into pieces increase if the capsule is pierced more than once during step 6.
Cleaning the Turbospin device
Clean the Turbospin inhaler after each dose using the following procedure:
- Press the piston all the way down a couple of times while keeping the chamber mouth downwards.
- Wipe the chamber with a cloth or cotton swab. Do not use water.
- Screw the mouthpiece firmly back into place, put the cap on and the inhaler will be ready to use for the next dose.
If you/your child uses more Colobreathe than you should, or if you have accidentally swallowed a capsule,contact your doctor immediately for help.
If you/your child misses a dose of Colobreathe
If you/your child forgets to take a dose of Colobreathe, then you/your child should take the missed dose as soon as you/your child remembers. You/your child should not take 2 doses in less than 12 hours. Continue from there as directed.
If you/your child stops treatment with Colobreathe
Do not stop your treatment prematurely unless your doctor tells you to. Your doctor will decide how long your treatment/your child’s treatment should last.
If you/your child has any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions
An allergic reaction with Colobreathe is possible (usually serious allergic reactions can cause skin rash, swelling of the face, tongue and throat, difficulty breathing due to narrowing of the airways and loss of consciousness). If you/your child experiences signs of an allergic reaction, you must seek urgent medical attention.
Other possible side effects
You/your child may have an unpleasant taste in your mouth after inhaling Colobreathe.
Very common (may affect more than 1 in 10 people):
- Difficulty breathing
- Cough, sore throat
- Hoarse or weak voice, or even loss of voice
- Unpleasant taste
Common (may affect up to 1 in 10 people):
- Headache
- Ringing or buzzing in the ears, balance problems.
- Coughing up blood, wheezing (a whistling sound that occurs when breathing), chest discomfort, asthma, productive cough (cough that produces mucus), lung infection, crackling in the lungs (your doctor hears this when listening to your lungs with a stethoscope)
- Vomiting, nausea
- Changes in your lung function (found in tests)
- Joint pain
- Lack of energy, tiredness
- Increased temperature
Uncommon (may affect up to 1 in 100 people):
- Allergic reactions (hypersensitivity); the signs may include rash and itching
- Weight fluctuations, decreased appetite
- Anxiety
- Seizures
- Drowsiness
- Ear blockage
- Chest pain
- Difficulty breathing
- Nosebleeds, runny nose (mucus in the nose, which can make you feel blocked), cough with thick green mucus, sore throat and sinuses
- Abnormal sounds in the chest (your doctor would hear this when listening to your lungs with a stethoscope)
- Diarrhoea, gas
- Excessive saliva production
- Toothache
- Protein in the urine (found in tests)
- Thirst
The side effects described above have been seen in people of all ages with a similar frequency.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Colobreathe
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer packaging and on the blister pack after EXP. The expiry date is the last day of the month shown.
Do not store Colobreathe above 25°C.
Store in the original package to protect from moisture.
If you/your child accidentally peels off the foil and a capsule is exposed, please discard that capsule.
Discard the Turbospin inhaler after completion of a treatment pack.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container contents and additional information
Composition of Colobreathe
The active ingredient is sodium colistimethate. Each capsule contains 1,662,500 IU (approximately equivalent to 125 mg) of sodium colistimethate.
The other components are:
Capsule shell
Gelatin
Polyethylene glycol
Sodium lauryl sulfate
Purified water
Appearance of the product and container contents
Colobreathe powder for inhalation, hard capsule (powder for inhalation) is supplied in small, hard, transparent gelatin capsules containing a fine white powder.
The Turbospin is a dry powder inhaler driven by the inspiratory flow, manufactured from polypropylene and stainless steel.
The capsules are packaged in blisters, which are supplied in boxes containing:
- 56 hard capsules and a Turbospin dry powder inhaler device, sufficient for 4 weeks of use.
- 8 hard capsules and a Turbospin dry powder inhaler device, sufficient for 4 days of use.
Only some pack sizes may be marketed.
Marketing authorization holder
Essential Pharma Limited,
Vision Exchange Building,
Territorials Street, Zone 1,
Central Business District,
Birkirkara, CBD 1070,
Malta
Manufacturer
Teva Pharmaceuticals Europe BV
Swensweg 5
2031 GA Haarlem
Netherlands.
Millmount Healthcare Limited
Block 7, City North Business Campus
Stamullen
Co Meath
K32 YD60
Ireland
Merckle GmbH
Ludwig-Merckle-Str-3
89143 Blaubeuren
Germany
Laboratorios Liconsa, S.A.
Miralcampo Avenue, 7, Industrial Estate
Miralcampo
19200 Azuqueca de Henares (Guadalajara)
Spain
Date of last revision of this leaflet: February 2024.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Colobreathe 1,662,500 IU powder for inhalation, hard capsulesDosage form: PULMONARY INHALATION, 1 MIUActive substance: colistinManufacturer: Pari Pharma GmbhPrescription requiredDosage form: PULMONARY INHALATION, 2 MIUActive substance: colistinManufacturer: Pari Pharma GmbhPrescription requiredDosage form: INJECTABLE, 1 IUActive substance: colistinManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for Colobreathe 1,662,500 IU powder for inhalation, hard capsules
Discuss questions about Colobreathe 1,662,500 IU powder for inhalation, hard capsules, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















