
COLIROFTA CICLOPLÉJICO 10 mg/ml EYE DROPS SOLUTION

How to use COLIROFTA CICLOPLÉJICO 10 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
COLIROFTA CICLOPLÉJICO 10 mg/ml eye drops, solution
cyclopentolate hydrochloride
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What COLIROFTA CICLOPLÉJICO is and what it is used for
- What you need to know before you use COLIROFTA CICLOPLÉJICO
- How to use COLIROFTA CICLOPLÉJICO
- Possible side effects
- Storage of COLIROFTA CICLOPLÉJICO
- Contents of the pack and other information
1. What is Colirofta Ciclopléjico and what is it used for
It is an eye drop that contains cyclopentolate hydrochloride as the active substance, which dilates the pupil (has a mydriatic effect) and paralyzes the eye's ability to focus (has a cycloplegic effect).
Colirofta Ciclopléjico is used for eye fundus examination and refraction test (to measure errors in eye focus and detect refractive defects such as myopia, astigmatism, presbyopia, etc.) and in any condition where a pupil dilation effect (mydriasis) or paralysis of the muscle that produces accommodation (cycloplegia) is desired, or when atropine (which has a similar effect with different action) cannot be used in inflammatory processes of the uveal tract or intermediate layer of the eye wall.
2. What you need to know before you use Colirofta Ciclopléjico
Do not use Colirofta Ciclopléjico:
- If you are allergic to cyclopentolate hydrochloride or any of the other components of this medicine (listed in section 6).
- If you have narrow-angle glaucoma (increased pressure in the eyes) or have narrow angles as a form.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Colirofta Ciclopléjico.
- Use this medicine only in your eye(s).
- You must be very careful if you have previously suffered a severe reaction affecting part of your body to the drug atropine, especially in children.
- Also, if you have a fever or are exposed to high temperatures, especially in children.
- Consult your doctor. The use of this medicine may cause:
- Increased pressure in the eye. Eye pressure should be checked before starting treatment, especially in elderly patients.
- Behavioral changes, especially in children and elderly patients, although these reactions can occur at any age.
- Sensitivity to light. Protect your eyes (with dark glasses) from intense light.
- If you are being treated with pilocarpine (for glaucoma), this medicine may interfere with that drug. Therefore, its use should be avoided at the same time.
Children
- This medicine is not recommended in newborns and infants (especially in premature and low-weight infants) due to the risk of severe adverse reactions. See also sections 3 and 4.
- It should be used with caution in children in general.
- Use with great caution in: premature infants, newborns, infants, young children or children with Down syndrome, with spastic paralysis (a condition that affects the muscles of the limbs), brain damage, known epilepsy. These children are very sensitive to suffering from nervous system, digestive, or heart and lung disorders due to absorption of the active substance into the body.
- Children with fair skin and blue eyes may be more prone to adverse effects.
- The use of this medicine may produce feeding intolerance and necrotizing enterocolitis (NEC) in premature infants. Cases of NEC have been reported in premature infants after administration. Therefore, newborns and infants should not be fed until 4 hours after administration.
- Avoid children putting this medicine in their mouth or cheeks. Wash your hands and the children's cheeks or hands immediately after administration.
Elderly patients
This medicine should be used with caution in elderly patients as they are more prone to increased pressure in the eyes.
Other medicines and Colirofta Ciclopléjico
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Especially, tell your doctor if you are using:
- Amantadine (used in Parkinson's disease and for the flu)
- Antihistamines (anti-allergics)
- Antipsychotics (for mental illnesses)
- Tricyclic antidepressants (for depression)
- Belladonna and its alkaloids, such as atropine and scopolamine (used before anesthesia or in digestive disorders, such as colic)
- Tiotropium (used by inhalation in respiratory diseases)
- Donepezil (used in Alzheimer's disease).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Colirofta Ciclopléjico is not recommended during pregnancy.
During breastfeeding, the doctor must decide whether it is necessary to interrupt breastfeeding or interrupt treatment with this medicine, considering the benefit of breastfeeding for the child and the benefit of treatment for the mother.
Driving and using machines
You may notice blurred vision and sensitivity to light for a prolonged period, such as 24 hours. Do not drive or use machines until these effects have disappeared.
COLIROFTA CICLOPLÉJICO contains methyl parahydroxybenzoate and propyl parahydroxybenzoate
It may cause allergic reactions (possibly delayed) because it contains methyl parahydroxybenzoate (E-218) and propyl parahydroxybenzoate (E-216).
3. How to use Colirofta Ciclopléjico
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
- To limit the amount of medicine that passes into the blood after applying the eye drops, keep your eyes closed while gently pressing the lacrimal canal with your finger for at least 2 minutes.
The recommended dose is:
Eye route.
Adults
Instill 1 or 2 drops in the eye(s) approximately 40-45 minutes before the refraction test. If necessary, this dose can be repeated after 5 or 10 minutes.
When a sustained effect is sought, 2 drops are usually instilled three times a day.
You should be cautious when using this medicine in elderly patients as they are more prone to increased pressure in the eyes.
Use in children
For refraction test:
- Children under 6 years: instill 1 drop of the solution, 40 or 50 minutes before the test. If necessary, a second drop can be instilled 5 or 10 minutes later.
In case of young children, see below.
- Children over 6 years: instill 1 or 2 drops of the solution; a second drop can be applied if necessary after 5 minutes, 40 or 50 minutes before the test.
After instillation, children should be carefully observed for 30 minutes.
You should be cautious when using this medicine in children (see section 2).
This medicine is not recommended in newborns and infants (especially in premature and low-weight infants) due to the risk of severe adverse reactions. See also the sections "Warnings and precautions" in section 2, "If you use more Colirofta Ciclopléjico than you should" in section 3, and section 4 "Possible side effects".
The use of this medicine in newborns and infants can produce feeding intolerance. Therefore, they should not be fed until 4 hours after administration.
Recommendations for use:
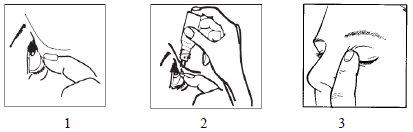
- Wash your hands.
- Take the bottle (dropper container).
- After opening the bottle for the first time, remove the plastic ring from the seal if it is loose.
- Hold the bottle, upside down, between your fingers.
- Tilt your head back. Gently separate the eyelid from the eye with your finger until a pocket forms between the eyelid and your eye, where the drop should fall (figure 1).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, or nearby areas or other surfaces with the dropper. The drops could become contaminated.
- Gently squeeze the base of the bottle with your index finger to release one drop at a time (figure 2).
- After using this eye drop, press the edge of the eye near the nose with your finger for at least 2 minutes. This helps prevent the eye drop from passing into the rest of the body (figure 3).
- If you are applying drops in both eyes, repeat all the previous steps with the other eye.
- Close the bottle tightly immediately after using the product.
- Remember to wash your hands after applying the eye drop.
If a drop falls outside the eye, try again.
If you are using other eye medicines, wait at least 5 minutes between the administration of this medicine and the other eye medicines. Eye ointments should be administered last.
If you use more Colirofta Ciclopléjico than you should
You can eliminate it by washing your eyes with warm water. Do not apply more drops until it is time for your next dose.
The symptoms of ocular overdose may include: redness and dryness of the skin (rash may occur in children), blurred vision, rapid and irregular pulse, fever, abdominal swelling in young children, convulsions, hallucinations, or loss of coordination in movements.
Symptomatic treatment should be provided.
The drug of choice is physostigmine.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used, as severe reactions (especially in children) may occur.
If you forget to use Colirofta Ciclopléjico
Do not apply a double dose to make up for forgotten doses.
Apply a single dose as soon as you remember and continue with the next dose that was scheduled. However, if it is almost time for the next dose, do not apply the forgotten dose and continue with the next dose of your regular regimen.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been observed:
Side effects of unknown frequency (cannot be estimated from the available data)
- Eye effects: sensitivity to light (photophobia), increased pupil size (prolonged effect of the medicine), eye irritation, blurred vision, eye pain, redness of the eye.
- General effects: allergy, hallucination, confusion, disorientation, agitation, restlessness, incoherence when speaking, loss of recent memory, dizziness, headache, drowsiness, vomiting, nausea, dry mouth, redness of the skin, difficulty walking, fever, and fatigue.
Other side effects:increased pressure in the eye and glaucoma in patients with a predisposition to angle closure, especially elderly patients; hyperactivity, tremor, anxiety, sedation, difficulty concentrating, increased heart rate, increased body temperature.
Other side effects in children
In addition, this medicine may produce in children lack of coordination, convulsions, skin rash, behavioral changes, psychiatric disorders, prolonged dilation of the pupils, abdominal swelling in infants, increased heart rate, dilation of blood vessels, urinary retention, decreased motility of the intestines, decreased secretion in the salivary and sweat glands, in the throat, in the bronchi, and nasal passages. Severe reactions are characterized by low blood pressure with shallow and rapid breathing.
In newborns and infants, the use of this medicine may produce feeding intolerance (see section 3). In premature infants, this medicine may produce necrotizing enterocolitis. In children, a local allergic reaction to this medicine has been observed, which consists of a skin rash.
The side effects of this medicine usually occur 20-30 minutes after application and although they are usually transient, symptoms can last from 12 to 24 hours.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es.By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Colirofta Ciclopléjico
Keep this medicine out of the sight and reach of children.
Do not store above 25°C.
Do not use this medicine after the expiry date which is stated on the bottle and carton after EXP. The expiry date is the last day of the month shown.
To avoid infections, the bottle should be discarded 4 weeks after it was first opened.
Write the date of opening on the space provided on the carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of COLIROFTA CICLOPLÉJICO
- The active substance is cyclopentolate hydrochloride. One ml of solution contains 10 mg of cyclopentolate hydrochloride (1%).
- The other components are: methyl parahydroxybenzoate (E-218), propyl parahydroxybenzoate (E-216), sodium chloride, and purified water.
Appearance and packaging of the product
Colirofta Ciclopléjico is an eye drop solution. It is a liquid (clear and colorless) that comes in a carton containing a dropper container (plastic bottle with a dropper dispenser) with a cap.
Each container holds 10 ml of eye drops.
Marketing authorization holder
Alcon Healthcare S.A.
World Trade Center Almeda Park
Plaça de la Pau s/n, Edificio 6, planta 3
08940 - Cornellà de Llobregat (Barcelona)
Spain
Manufacturer
Siegfried El Masnou, S.A.
C/Camil Fabra, 58
08320 – El Masnou (Barcelona)
Spain
or
Alcon Laboratories Belgium
Lichterveld 3
2870 Puurs-Sint-Amands
Belgium
Date of last revision of this leaflet:June 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price4.57 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COLIROFTA CICLOPLÉJICO 10 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, -Active substance: atropineManufacturer: Alcon Healthcare S.A.Prescription requiredDosage form: EYEDROP, 5 mgActive substance: atropineManufacturer: Alcon Healthcare S.A.Prescription requiredDosage form: EYEDROP, 10 mg/mlActive substance: tropicamideManufacturer: Alcon Healthcare S.A.Prescription required
Online doctors for COLIROFTA CICLOPLÉJICO 10 mg/ml EYE DROPS SOLUTION
Discuss questions about COLIROFTA CICLOPLÉJICO 10 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















