
Cikloftial

Ask a doctor about a prescription for Cikloftial

How to use Cikloftial
Package Leaflet: Information for the User
Cykloftyal, 10 mg/ml, Eye Drops, Solution
Cyclopentolate Hydrochloride
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Cykloftyal and what is it used for
- 2. Important information before using Cykloftyal
- 3. How to use Cykloftyal
- 4. Possible side effects
- 5. How to store Cykloftyal
- 6. Contents of the pack and other information
1. What is Cykloftyal and what is it used for
Cykloftyal eye drops contain cyclopentolate hydrochloride as the active substance at a concentration of 10 mg/ml.
Cyclopentolate hydrochloride is a substance that causes paralysis of the iris and ciliary muscle, leading to dilation of the pupil and paralysis of accommodation.
Cykloftyal is used:
- for eye exams and refraction tests in adults, adolescents, and children over 3 months old
- as a mydriatic agent in the treatment of uveitis, iritis, iridocyclitis, choroiditis, and retinal vasculitis in adults.
2. Important information before using Cykloftyal
When not to use Cykloftyal
- if you are allergic to cyclopentolate hydrochloride or any of the other ingredients of this medicine (listed in section 6);
- in elderly patients (over 65 years old);
- in children under 3 months old;
- in cases of narrow-angle glaucoma, as the use of the medicine may lead to the development of an acute condition;
- in children with organic brain changes (including congenital or developmental disorders of the nervous system, especially those predisposing to seizures).
Warnings and precautions
Before starting treatment with Cykloftyal, discuss it with your doctor or pharmacist.
Caution should be exercised in elderly patients due to the increased risk of systemic side effects, in patients with paralytic ileus, with benign prostatic hyperplasia, coronary insufficiency, or heart muscle damage, with ataxia, hypersensitivity to belladonna alkaloids, and with eye congestion (due to the possibility of increased systemic absorption).
Cyclopentolate hydrochloride increases the sensitivity of the eyes to light, so it is recommended to wear sunglasses to protect the eyes from ultraviolet radiation.
Cykloftyal should be used with caution in patients with glaucoma.
Full recovery of accommodation may take up to 24 hours.
Children and adolescents
Cykloftyal should not be used in newborns and infants under 3 months old due to the possible link between the induction of accommodation paralysis and the development of amblyopia, as well as the risk of systemic toxicity in newborns.
Caution is advised in children due to the risk of systemic effects and in cases of eye congestion, which may lead to increased systemic absorption.
Children with spastic paralysis or mental retardation are more prone to side effects of cyclopentolate hydrochloride.
Another risk, especially in children, is the absorption of cyclopentolate compounds through the nasal mucosa, where they enter the lacrimal canal with tear secretions. To limit the systemic absorption of cyclopentolate hydrochloride, after instillation into the eye, the lacrimal ducts (corner of the eye) should be compressed for about 2-3 minutes.
Cykloftyal and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
Medicines whose effects may be altered by Cykloftyal or that may affect its action include:
- long-acting cholinergic anti-glaucoma medications such as demecarium, ecotiopate, and isoflurophate,
- belladonna alkaloids
- carbachol
- pilocarpine
- amantadine
- certain antihistamines
- antipsychotic medications (phenothiazines, tricyclic antidepressants)
- monoamine oxidase inhibitors (MAOIs)
In the case of concurrent use of two ophthalmic medications, an interval of 5 to 10 minutes should be maintained between administrations.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, or if you plan to become pregnant or suspect you are pregnant, consult your doctor or pharmacist before using the medicine.
Since the active substance is absorbed into the body, pregnant and breastfeeding women may use Cykloftyal only when the doctor considers that the expected therapeutic benefits for the mother outweigh the potential risk to the child.
There are no results from animal studies assessing the risk of a negative impact on embryo and fetal development.
Driving and using machines
The medicine causes vision disturbances. Do not drive vehicles or operate machines after using the medicine.
Cykloftyal contains borate and benzalkonium chloride
Cykloftyal contains benzalkonium chloride (0.1 mg in 1 ml, 0.003 mg in one drop).
Benzalkonium chloride may be absorbed by soft contact lenses and change their color. Remove contact lenses before instillation and wait at least 15 minutes before reinserting.
Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or corneal disorders (the transparent layer at the front of the eye). If you experience any abnormal sensations in the eye, stinging, or pain in the eye after using the medicine, contact your doctor.
Cykloftyal contains boric acid (7.5 mg in 1 ml, 0.225 mg in one drop).
3. How to use Cykloftyal
Always use this medicine exactly as your doctor has told you.
In case of doubt, consult your doctor or pharmacist.
Adults under 65 years old
- Accommodation paralysis and eye exams: one drop of Cykloftyal should be instilled twice at 5-minute intervals. The exam should be performed about 30-40 minutes after the last instillation.
- Uveitis, iritis, iridocyclitis, choroiditis, and retinal vasculitis: one drop should be applied 3 to 4 times a day. The duration of treatment should be determined by the ophthalmologist based on clinical observation of the patient.
Adults over 65 years old: Use is contraindicated.
Children and adolescents
Children over 3 months old and adolescents:
- Accommodation paralysis and eye exams: one drop of Cykloftyal should be instilled into the eye 40 minutes before the exam. If necessary, the dose can be repeated after 15 minutes.
Children and adolescents should be observed for 30 minutes after instillation of Cykloftyal into the eye.
Resistance to accommodation paralysis may occur in young children (over 3 months to 6 years old), in patients with dark skin and/or dark irises, so there may be a need to adjust the dose in these groups.
Children under 3 months old:
Cykloftyal should not be used in newborns and infants under 3 months old due to the possible link between the induction of accommodation paralysis and the development of amblyopia, as well as the risk of systemic toxicity in newborns.
Method of administration:
Always wash your hands before using Cykloftyal and assume a comfortable position.
Cykloftyal should be applied as follows:
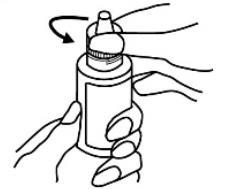
- 1. Open the bottle by unscrewing the cap, turning it counterclockwise;
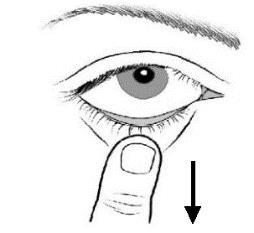
- 2. Tilt your head back and gently pull down the lower eyelid to create a small pocket between the eyelid and the eye;
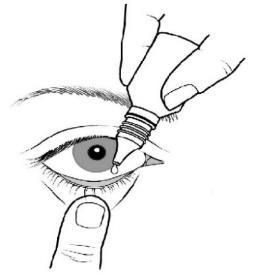
- 3. Invert the bottle and gently squeeze it so that one drop is instilled into the eye, according to the doctor's instructions. Do not touch the eye or eyelid with the dropper tip;
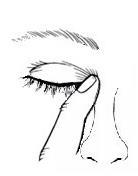
- 4. Close your eye, press your finger against the inner corner of your eye near your nose, and maintain the pressure for about 2-3 minutes with your eye closed. This prevents the medicine from entering the body;
- 5. If your doctor recommends using Cykloftyal in both eyes, steps 2-4 should be repeated for the other eye;
- 6. Immediately after use, tightly close the bottle cap. Do not overtighten the cap.
Using more than the recommended dose of Cykloftyal
In case of overdose, contact your doctor or the nearest hospital immediately, taking the Cykloftyal packaging with you.
Missing a dose of Cykloftyal
If the time to the next dose is far away, Cykloftyal should be used immediately. Then, the use of the medicine should be continued as recommended.
If, however, the time for the next dose is approaching, the missed dose should be skipped, and the medicine should be taken as recommended.
Do not use a double dose to make up for a missed dose.
In case of any further doubts about using the medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Cykloftyal can cause side effects, although not everybody gets them.
The frequency of side effects is defined as follows: very common (≥1/10), common (≥1/100 to <1>
- Local reactions that require contact with a doctor if they persist: Eye disorders Frequency not known: photophobia, eye burning (transient) after application, with repeated use, a potential development of an allergic reaction, which manifests as persistent eye irritation, blurred vision, and eye congestion, vision disturbances, increased intraocular pressure, blepharitis, conjunctivitis, punctate keratitis.
- Reactions resulting from systemic absorption and requiring contact with a doctor:
Frequency not known: confusion, hallucinations, unusual feeling of weakness or fatigue, fever, facial flushing, increased thirst or dry mouth, abdominal bloating, especially in children, skin rash, accelerated heart rate, urinary retention.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Cykloftyal
Keep the medicine out of the sight and reach of children.
Do not use the medicine after the expiry date stated on the carton and bottle after "EXP":
The expiry date refers to the last day of the month stated.
Shelf life after opening the bottle: 28 days
Do not store above 25°C.
Do not freeze.
Keep the bottle tightly closed.
Keep the bottle in the outer packaging to protect from light and moisture.
For ophthalmic use only. The dropper cannot be used for other purposes.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Cykloftyal contains
- The active substance is cyclopentolate hydrochloride
- The other ingredients are: boric acid, potassium chloride, disodium edetate, sodium carbonate, benzalkonium chloride, water for injections, sodium hydroxide 40% (for pH adjustment), hydrochloric acid 10% (for pH adjustment), sodium chloride
What Cykloftyal looks like and contents of the pack
Opaque white LDPE bottle with LDPE dropper and white HDPE cap with a tamper-evident seal in a cardboard box.
Each bottle contains 5 ml of solution.
Pack size: 1 x 5 ml.
The solution is clear and colorless.
Marketing authorization holder and manufacturer
Marketing authorization holder
Verco S.A.
Skwer kard. S. Wyszyńskiego 5, lok. 6U
01-015 Warsaw
Tel.: +48 22 811 41 61
Fax: +48 22 833 51 43
Manufacturer
Laboratório Edol – Produtos Farmacêuticos, S.A.
Av. 25 de Abril, n°. 6-6A
2795-225 Linda-a-Velha
Portugal
Laboratório Edol – Produtos Farmacêuticos, S.A.
Rua Quinta do Salrego 22-22A, Portela de Carnaxide, Carnaxide, 2790-144, Portugal and Rua Casal do Canas nº6-6A, Carnaxide, 2790-204, Portugal
Date of last revision of the leaflet:
XXXXXX.XX.XX
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLaboratorio Edol - Produtos Farmaceuticos, S.A. Laboratorio Edol - Produtos Farmaceuticos, S.A. Laboratorio Edol - Produtos Farmaceuticos, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CikloftialDosage form: Drops, 10 mg/mlActive substance: atropineManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription requiredDosage form: Drops, 100 mcg/mlActive substance: atropineManufacturer: Jadran-Galenski laboratorij d.d.Prescription requiredDosage form: Solution, (0.2 mg + 3.1 mg + 10 mg)/mlActive substance: tropicamide, combinationsManufacturer: Delpharm Tours Laboratoires THEAPrescription required
Alternatives to Cikloftial in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Cikloftial in Ukraine
Alternative to Cikloftial in Spain
Online doctors for Cikloftial
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Cikloftial – subject to medical assessment and local rules.














