

COLIRCUSI ANTIEDEMA 50 mg/mL EYE DROPS SOLUTION

Ask a doctor about a prescription for COLIRCUSI ANTIEDEMA 50 mg/mL EYE DROPS SOLUTION

How to use COLIRCUSI ANTIEDEMA 50 mg/mL EYE DROPS SOLUTION
Introduction
Package Leaflet: Information for the User
COLIRCUSÍ ANTIEDEMA 50 mg/ml eye drops, solution
Sodium chloride
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What COLIRCUSÍ ANTIEDEMA is and what it is used for
- What you need to know before using COLIRCUSÍ ANTIEDEMA
- How to use COLIRCUSÍ ANTIEDEMA
- Possible side effects
- Storage of COLIRCUSÍ ANTIEDEMA
- Package contents and additional information
1. What COLIRCUSÍ ANTIEDEMA is and what it is used for
It is an eye drop that contains sodium chloride as the active ingredient. Sodium chloride is in the form of a hypertonic solution, so that when it comes into contact with the corneal epithelium, it extracts water from the cornea. This helps to keep the cornea free of inflammation.
Colircusí Antiedema is indicated to reduce corneal edema (fluid accumulation in the cornea) that can be caused by various factors such as bullous keratopathy (small bumps that form on the cornea), eye surgery (especially corneal surgery), and hereditary corneal dystrophy or Fuchs' dystrophy (disorders in which abnormal material accumulates in the cornea).
2. What you need to know before using COLIRCUSÍ ANTIEDEMA
Do not use COLIRCUSÍ ANTIEDEMA:
- If you are allergic to sodium chloride or any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Colircusí Antiedema.
Use this medication only in your eye(s).
Children
The use of this medication is not recommended in children because there are currently no data available.
Other medications and Colircusí Antiedema
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
No interactions with this medication are known.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
In necessary cases, this medication can be used during pregnancy and breastfeeding.
Driving and using machines
You may notice that your vision becomes blurry for a while after applying this eye drop. Do not drive or use machines until this effect has disappeared.
COLIRCUSÍ ANTIEDEMA contains methyl sodium parahydroxybenzoate (E-219) and propyl sodium parahydroxybenzoate (E-217)
It can cause allergic reactions (possibly delayed) because it contains methyl sodium parahydroxybenzoate (E-219) and propyl sodium parahydroxybenzoate (E-217).
3. How to use COLIRCUSÍ ANTIEDEMA
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. If you have any doubts, consult your doctor or pharmacist again.
The recommended dose is:
Adults (including elderly patients):
Generally, 1 or 2 drops will be instilled into the affected eye every 3 or 4 hours.
The number of daily applications and the duration of treatment may be modified according to medical criteria.
Usage recommendations:
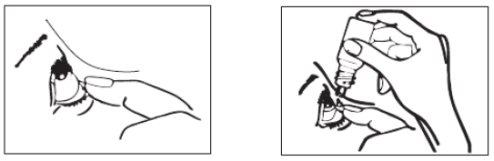
- Wash your hands.
- Take the bottle (or dropper bottle).
- After opening the bottle for the first time, remove the plastic ring from the seal if it is loose.
- Hold the bottle, upside down, between your fingers.
- Tilt your head back. Gently separate the eyelid of the eye with a finger until a pouch forms between the eyelid and your eye, where the drop should fall (figure 1).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, nearby areas, or other surfaces with the dropper. The drops may become contaminated.
- Gently squeeze the base of the bottle with your index finger to release one drop at a time (figure 2).
- If you are applying drops to both eyes, repeat all the previous steps with the other eye.
- Close the bottle immediately after using the product.
If a drop falls outside the eye, try again.
If you are using other eye medications, wait at least 5 minutes between the administration of this eye drop and other eye medications. Eye ointments should be administered last.
If you use more COLIRCUSÍ ANTIEDEMA than you should
An overdose in the eyes can be eliminated by rinsing the eyes with warm water. Do not apply more drops until it is time for your next dose.
In case of side effects due to an eye overdose, the symptoms may be similar to the local reactions reported with the use of the medication (see section 4).
No toxic effects are expected with the eye use of this medication or in the event of accidental ingestion of the contents of a bottle.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service. Telephone 91 562 04 20, indicating the medication and the amount used.
If you forget to use COLIRCUSÍ ANTIEDEMA
Do not apply a double dose to make up for forgotten doses.
Apply a single dose as soon as you remember and continue with the next dose that was scheduled. If it is almost time for the next dose, do not apply the forgotten dose and continue with the next dose of your regular regimen.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
The following side effects have been observed with this medication, but their frequency is unknown (cannot be estimated from the available data):
- Eye effects: eye pain, eye irritation, transient burning sensation.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Medication Monitoring System for Human Use: https://www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of COLIRCUSÍ ANTIEDEMA
Keep this medication out of the sight and reach of children.
Do not store at a temperature above 25°C.
Do not use this medication after the expiration date shown on the bottle and carton after EXP. The expiration date is the last day of the month indicated.
To avoid infections, the bottle should be discarded 4 weeks after it was first opened.
Write the opening date of the bottle in the space provided for this purpose on the carton.
Medications should not be thrown away in the drains or in the trash. Deposit the packaging and medications you no longer need at the SIGRE point in the pharmacy. If you have any doubts, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of COLIRCUSÍ ANTIEDEMA
- The active ingredient is sodium chloride. One milliliter of eye drops contains 50 mg of sodium chloride (5%).
- The other components are: methyl sodium parahydroxybenzoate (E-219), propyl sodium parahydroxybenzoate (E-217), hypromellose, and purified water.
Appearance of the product and package contents
Colircusí Antiedema is a colorless eye drop solution.
It is presented in a carton containing a dropper bottle (plastic bottle) with a screw cap. Each bottle contains 10 ml of eye drops.
Marketing authorization holder and manufacturer
Marketing authorization holder
OmniVision GmbH
Lindberghstrasse 9
82178 Puchheim
Germany
Manufacturer
Siegfried El Masnou, S.A.
C/ Camil Fabra, 58
08320 El Masnou – Barcelona, Spain
Local representative
OmniVision Farma España S.L.
C/ Josep Irla i Bosch, 1-3
Pl: 6 Pt: 2
08034 Barcelona
Spain
Date of the last revision of this package leaflet:January 2019.
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COLIRCUSI ANTIEDEMA 50 mg/mL EYE DROPS SOLUTIONDosage form: OPHTHALMIC OINTMENT, 50 mg sodium chloride/ gActive substance: sodium chloride, hypertonicManufacturer: Omnivision GmbhPrescription requiredDosage form: EYEDROP, 5.5 mg sodium chloride; 3 mg hypromellose/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Alcon Healthcare S.A.Prescription not requiredDosage form: EYEDROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not required
Alternatives to COLIRCUSI ANTIEDEMA 50 mg/mL EYE DROPS SOLUTION in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to COLIRCUSI ANTIEDEMA 50 mg/mL EYE DROPS SOLUTION in Poland
Alternative to COLIRCUSI ANTIEDEMA 50 mg/mL EYE DROPS SOLUTION in Ukraine
Online doctors for COLIRCUSI ANTIEDEMA 50 mg/mL EYE DROPS SOLUTION
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for COLIRCUSI ANTIEDEMA 50 mg/mL EYE DROPS SOLUTION – subject to medical assessment and local rules.














