
CITALOPRAM ARISTOGEN 40 mg FILM-COATED TABLETS

How to use CITALOPRAM ARISTOGEN 40 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Citalopram Aristogen 40 mg Film-Coated Tablets EFG
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
Package Leaflet Contents
- What is Citalopram Aristogen and what is it used for
- What you need to know before taking Citalopram Aristogen
- How to take Citalopram Aristogen
- Possible side effects
- Storage of Citalopram Aristogen
- Package Contents and Additional Information
1. What is Citalopram Aristogen and what is it used for
Citalopram Aristogen contains the active ingredient citalopram, which belongs to the group of selective serotonin reuptake inhibitors (SSRIs).
Citalopram Aristogen is an antidepressant and is used to treat depressive disorders and anxiety disorders with or without agoraphobia.
2. What you need to know before taking Citalopram Aristogen
Do not take citalopram
- If you are allergic to citalopram or any of the other ingredients of this medicine (listed in section 6).
- If you are taking monoamine oxidase inhibitors (MAOIs):
- E.g., the antidepressant moclobemide or if you are being treated with a non-selective MAOI - linezolid (an antibiotic), unless you are under close supervision and monitoring of your blood pressure.
- Irreversible MAOI inhibitor – selegiline (a medicine for Parkinson's disease), can be used in combination with citalopram in daily doses that do not exceed 10 mg of selegiline per day (see "Other medicines and citalopram").
- If you have taken irreversible MAOIs within the last two weeks or if you have taken reversible MAOIs (RIMAs) within the prescribed period in their corresponding prospectus (see "Other medicines and citalopram").
Warnings and precautions
Consult your doctor or pharmacist before starting to take this medicine.
Suicidal thoughts and worsening of depression or anxiety disorder
If you are depressed and/or suffer from anxiety disorders, you may sometimes have thoughts of self-harm or suicide. These can increase when taking antidepressants for the first time, as all these medicines need time to start working, usually around two weeks, but in some cases, the time could be longer.
You may be more prone to having these types of thoughts:
- If you have previously had thoughts of self-harm or suicide.
- If you are a young adult. Information from clinical trials has shown an increased risk of suicidal behavior in young adults, under 25 years, with psychiatric disorders who were being treated with an antidepressant.
If you have thoughts of self-harm or suicide at any time, contact your doctor or go to the hospital immediately.
It may be helpful to inform a family member or close friendthat you are depressed or have an anxiety disorder, and ask them to read this prospectus. You can ask them if they think your depression or anxiety disorder has worsened, or if they are concerned about changes in your attitude.
The following cases are described in which you should only take citalopram under certain conditions and with special caution. Consult your doctor about this. This also applies if this information applied to you in the past.
Do not take citalopram
- in patients who are being treated at the same time with other serotonergic medicines (e.g., tramadol, sumatriptan, or other triptans, oxitriptan, or tryptophan [serotonin precursors]). The simultaneous use can cause the so-called "serotonin syndrome". The possible signs are high fever, agitation, confusion, tremors, and muscle stiffness. In this case, consult your doctor immediately.
You should have special caution when taking citalopram if
- you suffer or have suffered heart problems or have recently had a heart attack;
- you have a low resting heart rate and/or know that you may have a decrease in salts as a result of intense and prolonged diarrhea and vomiting (feeling dizzy) or due to the use of diuretics (pills for dehydration);
- you experience a fast or irregular heartbeat, fainting, syncope, or dizziness when standing up, which may indicate abnormal heart rate function.
Tell your doctor if you have altered liver or kidney function. Your doctor may adjust the dose (see section 3).
In patients who suffer from epilepsy, treatment with citalopram should be discontinued if seizures occur or if their frequency increases (see also section 4, "Possible side effects").
Citalopram may also affect blood sugar control in patients with diabetes.
You may need to adjust your antidiabetic treatment.
During treatment with citalopram, rare cases of hyponatremia (low sodium levels in the blood) have been reported, possibly caused by inadequate secretion of antidiuretic hormone (SIADH), which was generally reversible after treatment discontinuation. Most reports referred to elderly patients, patients taking diuretics, or patients with volume deficit for other reasons. The signs may include discomfort with muscle weakness and confusion.
If you suffer from manic-depressive illness, you may experience a manic phase. If you enter a manic phase characterized by an unusual and rapid change of ideas, inappropriate happiness, and excessive physical activity, contact your doctor. In this case, treatment with citalopram should be suspended by your doctor.
Important information about your disease
Improvement does not occur immediately. After starting treatment with citalopram, it may take several weeks before you notice any improvement. In the treatment of anxiety disorder, it usually takes 2-4 weeks before noticing any improvement.
Some patients with anxiety disorder may experience an increase in anxiety at the beginning of treatment. However, these symptoms usually disappear on their own after 14 days of treatment. To reduce these anxiety symptoms, a low initial dose of 10 mg of citalopram is recommended during the first week of treatment (see section 3).
It is very important that you follow your doctor's instructions exactly and do not interrupt treatment or change the dose without consulting your doctor.
Medicines like citalopram (called SSRIs/SNRIs) may cause symptoms of sexual dysfunction (see section 4). In some cases, these symptoms persist after treatment discontinuation.
Restlessness/difficulty staying still or stopping
During the first weeks of treatment, symptoms such as restlessness, difficulty staying still, or stopping (akathisia) may appear. Inform your doctor immediately if you experience these symptoms. Then, a dose adjustment may be useful.
Withdrawal symptoms observed when stopping treatment with a Selective Serotonin Reuptake Inhibitor (SSRI)
When you stop treatment with citalopram, especially if it is sudden, you may feel withdrawal symptoms (see "How to take citalopram" and "Possible side effects"). They are common when treatment is stopped. The risk is greater when citalopram has been used for a long time, in high doses, or when the dose is reduced too quickly. Many people find that the symptoms are mild and disappear on their own within two weeks. However, in some patients, they can be severe or prolonged (2-3 months or more). If you experience severe withdrawal symptoms when stopping treatment with citalopram, please contact your doctor. You can ask them to start taking your tablets again and stop them more gradually.
In relation to the use of medicines like citalopram, rare cases of altered bleeding time and/or hemorrhagic disorders (e.g., ecchymosis, gynecological hemorrhages, gastrointestinal bleeding, and other cutaneous or mucous bleeding) have occurred.
Caution should be exercised in patients with a history of hemorrhagic disorders and during concomitant use of medicines that may increase the risk of bleeding (see also section "Other medicines and citalopram").
If you suffer from psychotic depression, treatment with citalopram may increase psychotic symptoms (e.g., hallucinations or delusions).
Currently, there is little clinical experience with the concomitant use of citalopram and electroconvulsive therapy, so caution is recommended.
Tell your doctor if you have eye problems such as certain types of glaucoma (increased intraocular pressure). Citalopram should be used with caution in this case.
Elderly patients (over 65 years)
Elderly patients are more sensitive to the effects of antidepressants, so the dose of citalopram should be adjusted by your doctor. Please inform your doctor if you experience any side effects.
Children and adolescents under 18 years
Citalopram should not be used normally in children and adolescents under 18 years. Moreover, you should know that patients under 18 years have a higher risk of suffering from adverse effects such as suicidal attempts, suicidal thoughts, and hostility (mainly aggressiveness, confrontational behavior, and anger) when taking this class of medicines.
Despite this, your doctor may prescribe citalopram to patients under 18 years when they decide what is most convenient for the patient. If your doctor has prescribed citalopram to a patient under 18 years and you want to discuss this decision, please go back to your doctor. You should inform your doctor if any of the symptoms listed above progress or worsen when patients under 18 years are taking citalopram. Also, the long-term effects on safety and related to growth, maturity, and cognitive and behavioral development of citalopram in this age group have not yet been demonstrated.
Other medicines and citalopram
Tell your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medicine.
Take only the medicines that your doctor has prescribed to you at the same time as citalopram.
Do not take citalopram
- If you are taking the so-called MAOIs (including selegiline in doses higher than 10 mg per day), as this combination can cause serious adverse effects (serotonin syndrome). There must be a sufficient time interval when switching between the two medicines. This change should only be made under strict medical control. You should not take citalopram during the two weeks following the discontinuation of treatment with irreversible MAOIs (e.g., tranilcipromine) and not before one day after discontinuing treatment with moclobemide (for the treatment of depression) or selegiline (for the treatment of Parkinson's disease). Treatment with MAOIs should not be started before one week after stopping citalopram. Serious and sometimes fatal reactions have occurred in patients who have received SSRIs with MAOIs (including moclobemide, linezolid, or selegiline).
- If you are taking linezolid.
- If you are taking medicines to treat heart rhythm problems or medicines that may affect heart rhythm, e.g., antiarrhythmic Class IA and III, antipsychotics (e.g., phenothiazine derivatives, pimozide, haloperidol), tricyclic antidepressants, certain antimicrobial agents (e.g., sparfloxacin, moxifloxacin, erythromycin IV, pentamidine, antimalarial treatment especially halofantrine), certain antihistamines (astemizole, mizolastine). If you have any questions about this, you should consult your doctor.
Citalopram should not be taken with sumatriptan and similar medicines (medicines for the treatment of migraine), tramadol (medicines for the treatment of pain), tryptophan, or oxitriptan (serotonin precursors), as it may cause an increase in the power of serotoninergic effects. There have also been isolated cases of "serotonin syndrome" (for its explanation, see section 4) when citalopram is combined with moclobemide or buspirone.
Concomitant administration of citalopram and herbal preparations containing St. John's Wort(herbal medicine for the treatment of depression) should be avoided due to the increased risk of adverse effects.
Concomitant administration of cimetidine, lansoprazole, and omeprazole (for the treatment of stomach ulcers), fluconazole (for the treatment of fungal infections), fluvoxamine (antidepressant), and ticlopidine (to reduce the risk of stroke) may cause an increase in the concentration of citalopram in the blood. Dose adjustment may be necessary.
Caution should be exercised when taking flecainide or propafenone (medicines used to treat irregular heart rhythm), desipramine, clomipramine, and nortriptyline (medicines used to treat depression) or risperidone, tioridazine, and haloperidol (medicines used to treat schizophrenia and psychosis) at the same time. Dose adjustment may be necessary.
Concomitant use of metoprolol (medicine for the treatment of high blood pressure and heart disease) causes an increase in the level of metoprolol in the blood. Dose adjustment may be necessary.
Although interactions with the concomitant use of citalopram and lithium (for the prevention and treatment of manic-depressive disorders) have been rarely reported, treatment should be carefully monitored.
Caution should be exercised when taking citalopram at the same time as other anticoagulant medicines (anticoagulants such as warfarin), medicines that affect platelet function, or other medicines that increase the risk of bleeding (see also "Warnings and precautions" in section 2). These medicines include non-steroidal anti-inflammatory drugs (ibuprofen, naproxen, acetylsalicylic acid, etc.), dipyridamole, antipsychotics, and ticlopidine.
Caution should be exercised when using medicines that decrease potassium or magnesium levels in the blood, as this increases the risk of suffering from heart rhythm disorders, which pose a risk to life.
Caution should be exercised when using imipramine and desipramine (both medicines used to treat depression) concomitantly. Dose adjustment of desipramine may be necessary.
Citalopram may decrease the seizure threshold. Therefore, caution should be exercised when treating concomitantly with medicines that also have a possible risk of decreasing the seizure threshold, e.g., mefloquine (used to treat malaria), bupropion (used to treat depression), tramadol (strong analgesic), neuroleptics (medicines for the treatment of schizophrenia or psychosis), and medicines for the treatment of depression (SSRIs).
Interactions between citalopram and clozapine (medicine used to treat psychosis) have been reported, which may increase the risk of adverse effects associated with clozapine. The nature of these interactions is not known.
Citalopram with alcohol
Although there is no evidence of interactions between citalopram and alcohol, alcohol consumption should be avoided during treatment with this medicine.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
Do not take citalopram if you are pregnant or plan to become pregnant, unless you and your doctor have discussed the risks and benefits.
Do not stop treatment with citalopram abruptly during pregnancy. Consult your doctor if you want to stop or interrupt treatment.
Make sure your midwife and/or doctor knows that you are being treated with citalopram. When medicines like citalopram are taken during pregnancy, particularly in the last three months of pregnancy, the risk of suffering from a serious disorder in newborns, called persistent pulmonary hypertension of the newborn (PPHN), may be increased, which makes the baby breathe more rapidly and have a bluish appearance. These symptoms usually appear within the first 24 hours after the baby's birth. If this happens to your baby, you should contact your midwife and/or doctor immediately.
If you are taking citalopram during the last three months of pregnancy and up to the day of your child's birth, you may experience serious adverse effects or withdrawal, such as respiratory difficulties, blue-tinged skin/lips, irregular breathing with breathing pauses, temperature fluctuations, seizures, apathy, difficulty sleeping, difficulty feeding, vomiting, low blood sugar levels, stiff or flexible muscles, increased reflexes, tremors, extreme nervousness or agitation, irritability, constant crying, and drowsiness.
If your newborn baby suffers from any of these symptoms, contact your doctor immediately; they will be able to advise you.
If you take citalopram in the final stage of pregnancy, there may be an increased risk of abundant vaginal bleeding shortly after delivery, especially if you have a history of hemorrhagic disorders. Your doctor or midwife should know that you are taking citalopram to be able to advise you.
Breastfeeding
Citalopram is excreted in breast milk in small amounts. There is a risk of effects on the child. If you are taking citalopram, inform your doctor before starting breastfeeding.
Fertility
Citalopram has been shown to reduce sperm quality in animals. This could theoretically affect fertility, but so far, no effect on fertility has been observed in humans.
Driving and using machines
Citalopram has a mild to moderate influence on the ability to drive and use machines. Do not drive or use machines until you know how this medicine affects you. Normally, citalopram does not affect the ability to perform daily activities. However, if you feel dizzy or drowsy when you start taking this medicine, you should be careful when driving, operating machinery, or performing tasks that require you to be alert until these effects disappear. If you are not sure, consult your doctor if you can perform the aforementioned activities.
Citalopram Aristogen contains lactose
This medicine contains lactose. If your doctor has told you that you have an intolerance to certain sugars, consult them before taking this medicine.
Citalopram Aristogen contains sodium
This medicine contains sodium.
3. How to take Citalopram Aristogen
Follow the administration instructions for this medication exactly as indicated by your doctor. In case of doubt, consult your doctor again.
Unless otherwise prescribed by a doctor, the usual dose is:
Adults:
For the treatment of depression
The recommended dose is 20 mg per day. Your doctor may increase this dose up to a maximum of 40 mg per day.
For the treatment of anxiety disorder with or without agoraphobia
The initial dose is 10 mg of citalopram per day for the first week, before increasing the dose to 20-30 mg of citalopram per day. This dose may be increased by your doctor up to a maximum of 40 mg of citalopram per day.
Elderly patients (over 65 years old)
Treatment should be started with half the recommended dose, e.g., 10-20 mg per day. Elderly patients should not normally take more than 20 mg per day.
Use in patients with special risks
In patients with impaired liver function, the elimination of citalopram may be slow. In mild to moderate hepatic insufficiency, an initial dose of 10 mg of citalopram per day is recommended for the first two weeks of treatment. Patients with hepatic insufficiency should not take more than 20 mg of citalopram per day. Your doctor should monitor your liver function. Caution and extremely careful dosing are recommended if you have severe liver problems.
No dose adjustment is necessary in case of mild or moderate renal insufficiency. The use of citalopram is not recommended in patients with severe renal insufficiency (creatinine clearance less than 30 ml/min) due to lack of experience.
Use in children and adolescents under 18 years old
Citalopram should not normally be used in children and adolescents under 18 years old (see "Warnings and precautions").
How and when should you take citalopram?
Citalopram is for oral use.
The tablets are taken once a day.
The tablets can be taken at any time of day, regardless of meals, with plenty of liquid (e.g., 1 glass of water). Do not chew the tablets because they have a bitter taste.
The tablet can be divided into equal doses.
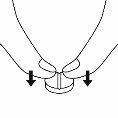 Divisibility warning
Divisibility warning
Place the tablet with the marked line facing up on a flat, hard surface.
To divide the tablet, press it from top to bottom on the left and right of the marked line with your thumb or index finger.
Division should not be performed with a pill cutter.
How long should you take citalopram?
Note that the effect of citalopram may take several weeks. It may take 2 to 4 weeks before you start to feel better. Therefore, expect an improvement only after some time.
In the treatment of anxiety disorders with and without agoraphobia, the maximum effect is achieved after about three months of treatment.
The total duration of treatment varies greatly from person to person (usually at least 6 months) and is determined by your doctor. Respect this time even if you already feel better or are symptom-free, to avoid a new worsening or relapse of the disease.
If you take more citalopram than you should
If you or someone else has taken a quantity greater than the prescribed dose, inform your doctor immediately or go to the emergency department of the nearest hospital. Do this even if you have no symptoms and take the citalopram package with you.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount taken.
In case of overdose, the following symptoms may appear: drowsiness, numbness, loss of consciousness, convulsions, dizziness, nausea, vomiting, cyanosis (bluish discoloration of the skin), rapid and deep breathing (hyperventilation), tremors, sweating, agitation, dilated pupils, serotonin syndrome (see section 4), potentially fatal cardiac rhythm disturbances, acceleration or slowing of heart rate, increase or decrease in blood pressure, changes in the ECG, cardiac arrest, and dissolution of muscle fibers (rhabdomyolysis).
If you forget to take citalopram
If you forgot to take citalopram once, take the medication as usual the next time and consult your doctor.
Do not take a double dose to make up for forgotten doses.
If you interrupt treatment with citalopram
If you wish to interrupt treatment, discuss it with your doctor beforehand, as they will take the necessary measures if necessary. Do not stop taking this medication without consulting your doctor.
Withdrawal symptoms when stopping treatment with citalopram
Abrupt interruption of treatment should be avoided. When stopping treatment with citalopram, the dose should be gradually reduced over a period of at least one or two weeks to reduce the risk of withdrawal symptoms. If very harmful withdrawal symptoms occur, especially if it has been used for a long time in high doses or when the dose is reduced too quickly, it should be considered to resume the last dose and then reduce it in smaller increments according to the doctor's instructions (see sections 2 and 4).
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible adverse effects
Like all medications, this medication can cause adverse effects, although not all people experience them.
Adverse effects usually disappear after a few weeks of treatment. Several of the effects listed below may also be symptoms of your illness and may disappear when you start to feel better.
The following adverse effects can have serious consequences. If you experience any of the following symptoms, stop taking this medication and consult your doctor immediately:
- High fever, agitation, confusion, tremors, and sudden muscle contractions may be signs of a rare condition called "serotonin syndrome", which occurs when a combination of antidepressants is taken.
- Swelling of the skin, tongue, lips, or face, difficulty breathing or swallowing (allergic reaction),
- unusual bleeding, including gastrointestinal bleeding,
- rapid and irregular heartbeat, fainting. These may be symptoms of a potentially fatal heart rhythm disorder called Torsade de Pointes.
- discomfort with muscle weakness and confusion or difficulty urinating are signs of a rare disease called hyponatremia (low sodium levels in the blood), which can occur during treatment with SSRIs (a group of antidepressants that includes citalopram), especially in elderly women, patients taking diuretics, or patients with volume deficit for other reasons.
- seizures, see also "Warnings and precautions",
- yellowish discoloration of the skin and the white part of the eyes are symptoms of liver dysfunction/hepatitis.
The following adverse effects may occur:
Very common adverse effects (may affect more than 1 in 10 people)
- drowsiness;
- insomnia;
- tremors;
- nausea, constipation;
- increased sweating, dry mouth, feeling of weakness;
- headache.
Common adverse effects (may affect up to 1 in 10 people)
- nervousness, anxiety, agitation, abnormal dreams, apathy, decreased appetite, weight loss, confusion, difficulty concentrating;
- dizziness, attention disorders, sensation of tingling in hands and feet, such as numbness (paresthesia), movement disorders and involuntary movements (EPS disorders);
- diarrhea, vomiting, abdominal pain, indigestion, flatulence;
- rhinitis;
- sexual dysfunction, such as ejaculation disorders, lack of ejaculation, impotence, decreased sexual behavior, female orgasm disorders;
- taste disorders, visual disturbances, ringing in the ears (tinnitus);
- skin rash, itching;
- muscle pain, joint pain;
- fever;
- difficulty urinating;
- decrease in blood pressure and dizziness when standing up, palpitations, rapid heartbeat;
- increased salivation, yawning, alteration of general condition, fatigue.
Uncommon adverse effects (may affect up to 1 in 100 people)
- allergic reaction, hives;
- increased appetite, weight gain;
- euphoria, aggression, feeling of unreality, hallucinations, mania;
- seizures;
- cough, difficulty breathing;
- hypersensitivity to light;
- increased levels of liver enzymes;
- slow heart rate;
- fainting;
- hair loss;
- dilated pupils;
- women: prolonged menstrual bleeding;
- skin bleeding (purpura);
- swelling of arms and legs (edema).
Rare adverse effects (may affect up to 1 in 1,000 people)
- decrease in sodium levels in the blood (symptoms may include discomfort with muscle weakness and confusion or difficulty urinating);
- increase in urine production (abnormal secretion of antidiuretic hormone);
- serotonin syndrome (possible symptoms include high fever, agitation, confusion, and tremors and sudden muscle contractions);
- grand mal seizures;
- liver inflammation (hepatitis);
- inability to remain still (see section 2);
- involuntary movements (dyskinesia);
- skin or mucous membrane bleeding (ecchymosis);
- bleeding.
Frequency not known (cannot be estimated from available data):
- Suicidal thoughts and behaviors
- There have been reports of suicidal thoughts and behaviors during treatment with citalopram or just after stopping treatment (see section 2)
- Prolongation of the QT interval in the ECG (variations in heart rhythm).
- There have been reports of QT prolongation after marketing, predominantly in patients with pre-existing heart conditions.
- Rapid and irregular heartbeats, fainting. These may be symptoms of a potentially fatal heart rhythm disorder called Torsade de Pointes;
- increased prolactin levels;
- decrease in blood platelet levels, which increases the risk of bleeding and bruising;
- decrease in blood potassium levels (signs may be muscle weakness, spasms, and cardiac arrhythmias);
- pancreatitis;
- panic attacks;
- nocturnal teeth grinding;
- restlessness;
- secretion of milk in men and in non-lactating women;
- women: irregular menstrual bleeding, abundant vaginal bleeding shortly after childbirth (postpartum hemorrhage); for more information, see "Pregnancy, lactation, and fertility" in section 2;
- men: painful erections;
- sudden swelling of the skin or mucous membranes;
- nosebleeds;
- gastrointestinal bleeding (including rectal bleeding);
- severe allergic reaction (anaphylactic), with difficulty breathing and dizziness;
- movement disorders.
An increased risk of bone fractures has been observed in patients treated with this group of medications.
Withdrawal symptoms when stopping treatment with citalopram
Withdrawal symptoms usually occur when treatment is stopped. Dizziness, sensory disturbances (including paresthesias), sleep disturbances (including insomnia and intense dreams), agitation or anxiety, nausea and/or vomiting, tremors, confusion, sweating, headache, diarrhea, palpitations, emotional instability, irritability, and visual disturbances are the most frequently reported reactions.
These symptoms are usually mild to moderate and resolve on their own; however, in some patients, they can be severe and persist for a longer period. Therefore, it is recommended to gradually reduce the dose when treatment with citalopram is no longer necessary (see sections 2 and 3).
Reporting adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System for Human Use www.notificaram.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Citalopram Aristogen
Keep this medication out of sight and reach of children.
This medication does not require special storage conditions.
Do not use this medication after the expiration date that appears on the bottle and carton after EXP. The expiration date is the last day of the month indicated.
Medications should not be thrown down the drain or into the trash. Deposit the packages and medications you no longer need in the SIGRE point of the pharmacy. If in doubt, ask your pharmacist how to dispose of the packages and medications you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Citalopram Aristogen
- The active ingredient is citalopram.
- Each film-coated tablet contains 40 mg of citalopram (as hydrobromide)
- The other components (excipients) are:
- Core: microcrystalline cellulose, lactose monohydrate, calcium phosphate dihydrate, sodium carboxymethyl starch type A (potato), anhydrous colloidal silica, magnesium stearate.
- Coating: calcium carbonate, hypromellose, macrogol 6000.
Appearance of the product and package contents
White to off-white, round, biconvex film-coated tablets, engraved with "40" on one face and with a score line on the same face, with a diameter of approximately 8 mm.
The tablet can be divided into equal doses.
PVC/Aluminum blister packs containing 10, 14, 20, 30, 50, 100 tablets, and 250 tablets (clinical package).
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Aristo Pharma GmbH
Wallenroder Straße 8-10
13435 Berlin, Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Aristo Pharma Iberia, S.L.
C/ Solana, 26
28850, Torrejón de Ardoz
Madrid, Spain
This medication is authorized in the Member States of the European Economic Area under the following names:
Germany Citalopram Aristo 40 mg film-coated tablets
Austria Citalopram Aristo 40 mg film-coated tablets
Spain Citalopram Aristogen 40 mg film-coated tablets EFG
Portugal Citalopram Aristogen
Date of the last revision of this leaflet:May 2024
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CITALOPRAM ARISTOGEN 40 mg FILM-COATED TABLETSDosage form: TABLET, 20 mgActive substance: citalopramManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 30 mgActive substance: citalopramManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 10 mgActive substance: citalopramManufacturer: Aristo Pharma GmbhPrescription required
Online doctors for CITALOPRAM ARISTOGEN 40 mg FILM-COATED TABLETS
Discuss questions about CITALOPRAM ARISTOGEN 40 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











