
CEZIBOE 0.25 mg Injectable Solution in Pre-filled Syringe

How to use CEZIBOE 0.25 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Ceziboe 0.25 mg solution for injection in pre-filled syringe
cetrorelix (as acetate)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Ceziboe is and what it is used for
- What you need to know before you use Ceziboe
- How to use Ceziboe
- Possible side effects
- Storage of Ceziboe
- Contents of the pack and other information
1. What Ceziboe is and what it is used for
What Ceziboe is
Ceziboe contains a substance called “cetrorelix acetate”. This substance prevents your body from releasing an egg from the ovary (ovulation) during your menstrual cycle. Ceziboe belongs to a group of medicines known as “gonadotropin-releasing hormone antagonists”.
What Ceziboe is used for
Ceziboe is one of the medicines used during “assisted reproduction techniques” to help you get pregnant. It works by immediately stopping the release of eggs. This is because if the eggs are released too early (premature ovulation), your doctor may not be able to collect them.
How Ceziboe works
Ceziboe blocks a natural hormone in your body, called LHRH (“luteinizing hormone-releasing hormone”).
- LHRH controls another hormone, called LH (“luteinizing hormone”).
- LH stimulates ovulation during the menstrual cycle.
This means that Ceziboe stops the chain of events that leads to the release of an egg from the ovary. When your eggs are ready to be collected, you will be given another medicine that will release them (induction of ovulation).
2. What you need to know before you use Ceziboe
Do not use Ceziboe
- if you are allergic to cetrorelix (as acetate) or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to medicines similar to Ceziboe (any other peptide hormone).
- if you are pregnant or breast-feeding.
- if you have severe kidney disease.
Do not use Ceziboe if any of the above conditions apply to you. If in doubt, consult your doctor before using this medicine.
Warnings and precautions
Allergies
Consult your doctor before using Ceziboe if you have an active allergy or have had allergies in the past.
Ovarian Hyperstimulation Syndrome (OHSS)
Ceziboe is used together with other medicines that stimulate your ovaries to develop more eggs ready to be released. During or after receiving these medicines, you may suffer from OHSS. This situation occurs when your follicles develop too much and become large cysts.
To learn about the possible signs to look out for and what to do if they occur, see section 4 “Possible side effects”.
Use of Ceziboe for more than one cycle
Experience with the use of Ceziboe for more than one cycle is limited. Your doctor will carefully evaluate the benefits and risks for you if you need to use Ceziboe for more than one cycle.
Liver disease
Tell your doctor before using Ceziboe if you have any liver disease. Ceziboe has not been studied in patients with liver disease.
Kidney disease
Tell your doctor before using Ceziboe if you have any kidney disease. Ceziboe has not been studied in patients with kidney disease.
Children and adolescents
Ceziboe is not indicated for use in children and adolescents.
Other medicines and Ceziboe
Tell your doctor if you are using, have recently used or might use any other medicines.
Pregnancy and breast-feeding
Do not use Ceziboe if you are pregnant, think you may be pregnant or if you are breast-feeding.
Driving and using machines
Ceziboe is not expected to affect your ability to drive and use machines.
3. How to use Ceziboe
Follow the instructions for administration of this medicine exactly as told by your doctor. If in doubt, consult your doctor again.
Use of this medicine
This medicine is for subcutaneous injection only, into the skin of your abdomen. To reduce skin irritation, choose a different area of your abdomen each day.
- Your doctor should supervise the first injection. Your doctor or nurse will teach you how to prepare and inject the medicine.
- You can put in the following injections yourself, as long as your doctor has informed you of the symptoms that may indicate an allergy and the possible serious or life-threatening consequences that require immediate treatment (see section 4 “Possible side effects”).
- Read and follow carefully the instructions at the end of this leaflet, titled “How to inject Ceziboe”.
- You will start using another medicine on day 1 of your treatment cycle. Then, you will start using Ceziboe a few days later. (See the next section “How much to use”.)
How much to use
Inject the contents of one syringe (0.25 mg of Ceziboe) once a day. It is best to use the medicine at the same time each day, leaving 24 hours between each dose.
You can choose to do the injection either in the morning or at night.
- If you do the injection in the morning: Start your injections on the fifth or sixth day of your treatment cycle. Depending on your ovarian response, your doctor may decide to start treatment on another day. Your doctor will tell you the exact date and time. You will keep using this medicine until the morning your eggs are collected, including that morning (induction of ovulation).
OR
- If you do the injection at night: Start your injections on the fifth day of your treatment cycle. Depending on your ovarian response, your doctor may decide to start treatment on another day. Your doctor will tell you the exact date and time. You will keep using this medicine until the night before your eggs are collected, including that night (induction of ovulation).
If you use more Ceziboe than you should
No side effects are expected if you accidentally inject more medicine than you should. The effect of the medicine will last longer. In general, no specific measures are necessary.
In case of overdose or accidental ingestion, contact your doctor or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount taken.
If you forget to use Ceziboe
- If you forget a dose, inject it as soon as you remember and consult your doctor.
- Do not inject a double dose to make up for forgotten doses.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions
- Redness and feeling of heat in the skin, itching (often in the groin or armpits), red, swollen, and itchy areas (urticaria), runny nose, rapid or irregular heartbeat, swelling of the tongue and throat, sneezing, whistling sound while breathing (wheezing), severe difficulty breathing or dizziness. You may be suffering from a possible serious allergic reaction to the medicine, which can be life-threatening. This is uncommon (may affect up to 1 in 100 women).
If you notice any of the above side effects, stop using Ceziboe and consult your doctor immediately.
Ovarian Hyperstimulation Syndrome (OHSS)
This can occur due to the other medicines you are using to stimulate your ovaries.
- Pain in the lower abdomen, along with feeling of nausea or vomiting, can be symptoms of OHSS. This may indicate that your ovaries have overreacted to treatment and have developed large ovarian cysts. This effect is common (may affect up to 1 in 10 women).
- OHSS can become severe, with clearly enlarged ovaries, decreased urine production, weight gain, difficulty breathing, or fluid accumulation in the stomach or chest. This effect is uncommon (may affect up to 1 in 100 women).
If you notice any of the above side effects, consult your doctor immediately.
Other side effects
Common (may affect up to 1 in 10 women):
- Mild and short-lasting skin reactions may occur at the injection site, such as redness, itching, or swelling.
Uncommon (may affect up to 1 in 100 women):
- Feeling of nausea.
- Headache.
Reporting of side effects

If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ceziboe
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the syringe after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C).
The unopened product can be stored in the original packaging at room temperature (not above 25°C) for a maximum of three months.
Do not use this medicine if you notice that the solution in the syringe is not clear and colorless or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Ceziboe
- The active substance is cetrorelix (as acetate). Each pre-filled syringe (1 ml) contains 0.25 mg of cetrorelix (as acetate).
- The other ingredients are mannitol (E421), s-lactic acid, and water for injections.
Appearance and packaging
Ceziboe is a clear, colorless solution in a type I glass syringe (1 ml), fixed to a 27G x 12 mm needle, closed with a bromobutyl rubber stopper. The pre-filled syringe has a white plunger and an automatic safety system.
It is available in packs of 1 pre-filled syringe and in multipacks containing 7 cartons, each with 1 pre-filled syringe.
For each syringe, the packaging also contains:
- a pre-filled syringe with a safety device containing the solution
- a swab soaked in alcohol for skin cleaning.
Not all pack sizes may be marketed.
Marketing authorisation holder
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Netherlands
Manufacturer
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Netherlands
S.C. Terapia S.A.
124 Fabricii Street
400632, Cluj-Napoca
Cluj County
Romania
You can ask for more information about this medicine from the local representative of the marketing authorisation holder:
Sun Pharma Laboratories, S.L.
Rambla de Catalunya, 53-55
08007- Barcelona
Spain
Tel.: +34 93 342 78 90
This medicine is authorised in the Member States of the European Economic Area under the following names:
Belgium Ceziboe
Czech Republic Ceziboe
Germany Ceziboe
Greece Ceziboe
France Ceziboe
Hungary Ceziboe
Ireland Ceziboe
Italy Ceziboe
Netherlands Ceziboe
Spain Ceziboe
United Kingdom Ceziboe
Date of last revision of this leaflet: November 2020
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products: http://www.aemps.gob.es
HOW TO INJECT CEZIBOE
The different parts of the Ceziboe pre-filled syringe are
- Rigid needle cap
- Plunger
- Finger grip
- Safety protector
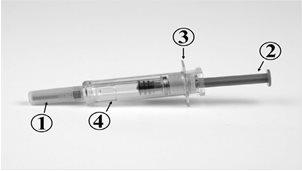
Syringe before use:
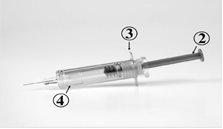
Syringe after use:

This section explains how to inject your medicine.
- Before you start using this medicine, read these instructions completely.
- This medicine is for you only; do not let anyone else use it.
- Use each needle and syringe only once.
Before you start
- This medicine should be at room temperature before injection. Take it out of the refrigerator approximately 30 minutes before use.
- Wash your hands.
It is important that your hands and the objects you use are as clean as possible.
3.
- A pre-filled syringe.
- An alcohol swab.
Preparing the injection site and injecting the medication
- Clean the injection site
- Sit or lie down in a comfortable position. Choose an injection site on your stomach. The area around the navel is best (Figure A).
To reduce skin irritation, select a different part of your stomach each day. Clean the injection site with an alcohol swab
- Remove the needle cap by pulling it straight off the syringe (Figure B). Discard the needle cap . To prevent infections, do not touch the needle or let it come into contact with any surface before injection. A small air bubble in the syringe is normal. To ensure you do not lose any medication from the syringe, do not attempt to remove air bubbles from the syringe before administering the injection.
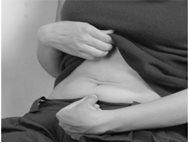

Figure A Figure B
- Gently pinch the skin you have cleaned to form a fold. Hold the fold between the thumb and index finger of one hand throughout the injection (Figure C).
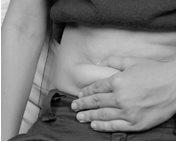
Figure C
- Hold the syringe with one hand, as if it were a pencil.
Gently pinch the skin around the injection site and hold it firmly with the other hand.
Slowly push the needle completely into your skin at an angle of approximately 45 to 90 degrees, then release your skin.
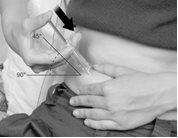
Figure D
- Gently pull back on the syringe plunger. If blood appears, continue as indicated in step 5. If no blood appears, slowly push the plunger to inject the medication. Inject all the medication in the syringe by gently pressing the plunger until it stops (Figure E). Warning: Do not press firmly, as this will activate the needle safety shield, see step 6.
When the syringe is empty, slowly withdraw the needle at the same angle, keeping your finger on the plunger. Use your alcohol swab to apply gentle pressure where you just injected.
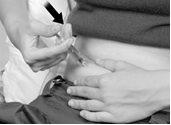
Figure E
- If blood appears:
Slowly withdraw the needle at the same angle. Use the alcohol swab to apply gentle pressure to the skin puncture site. Empty the medication into a sink and continue as indicated in step 6 below. Wash your hands and start again with a new pre-filled syringe (Figure F).
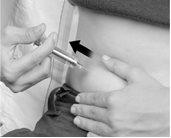
Figure F
- Point the needle away from you and others, and activate the safety shield by firmly pressing the plunger. The protective sleeve will automatically cover the needle, and you will hear an audible "click" to confirm shield activation (Figure G).
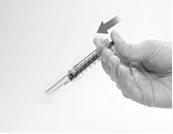
Figure G
Follow the instructions given by your nurse or doctor on how to dispose of used syringes and needles. There may be national laws on the correct way to dispose of used syringes, needles, and disposal containers.
NOTE:
- The Safety System can only be activated once the syringe has been emptied.
- Activation of the Safety System must be done only after the needle has been removed from the patient's skin.
- Do not replace the needle shield after injection.
- The Safety System must not be sterilized.
- Activating the Safety System may cause a minimal liquid splash. For optimal safety, activate the system while pointing it downwards, away from you and others.
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CEZIBOE 0.25 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 0.25 mgActive substance: cetrorelixManufacturer: Intas Third Party Sales 2005 S.L.Prescription requiredDosage form: INJECTABLE, UnknownActive substance: cetrorelixManufacturer: Merck Europe B.V.Prescription requiredDosage form: INJECTABLE, UnknownActive substance: cetrorelixManufacturer: Merck Europe B.V.Prescription required
Online doctors for CEZIBOE 0.25 mg Injectable Solution in Pre-filled Syringe
Discuss questions about CEZIBOE 0.25 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






