
CARDI-BRAUN MAINTENANCE SOLUTION FOR INFUSION

How to use CARDI-BRAUN MAINTENANCE SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet:information for theuser
Cardi-Braun Maintenance solution for perfusion
Read the entire leaflet carefully before starting to usethis medication.
- Keep this leaflet, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist
- If you consider that any of the adverse effects you are suffering from is serious or if you notice any adverse effect not mentioned in this leaflet, inform your doctor or pharmacist.
Contents of the leaflet:
- What is Cardi-Braun Maintenance and what is it used for
- Before using Cardi-Braun Maintenance
- How to use Cardi-Braun Maintenance
- Possible side effects
- Storage of Cardi-Braun Maintenance
- Additional information
1. What is Cardi-Braun Maintenance and what is it used for
The medications Cardi-Braun Maintenance and Cardi-Braun Reperfusion are solutions for intracardiac perfusion (administered directly to the heart through an external device that assumes the functions of the heart) that belong to the group of medications called cardioplegic solutions. The two solutions are used alternately during heart operations.
The cardioplegic solutions of B. Braun Medical are used in open-heart surgery to:
- stop the heart and protect the heart muscle during the operation,
- effectively resuscitate the heart function once the operation is finished.
2. Before using Cardi-Braun Maintenance
Do not use Cardi-Braun Maintenance
If you are allergic (hypersensitive) to the active ingredients or to any of the other components of Cardi-Braun Maintenance.
If, in your clinical situation, the technique of stopping the heart with cardioplegic solutions may pose a threat to your life.
Be careful with Cardi-Braun Maintenance
The cardioplegic solutions Cardi-Braun will only be used by qualified cardiac surgeons and specialists.
The cardioplegic solutions Cardi-Braun are not administered intravenously, they are released directly into the heart through an external device that assumes the functions of the heart during the operation.
During the operation, the medical team that assists you will carefully monitor your vital signs.
In particular, they will evaluate:
- the temperature of your heart,
- the electrocardiogram (measures the electrical activity of the heart) and
- the pressure and speed at which these solutions are administered.
For the recovery of the heart once the operation is finished, a defibrillation team (uses electric current to restore the heartbeat) and medications that increase the strength of the heart's contraction must be available.
Adequate intake of vitamins (particularly vitamin B1) must be guaranteed
Use of other medications
Certain medications may influence the action of the Cardi-Braun solutions. In these cases, it may be necessary to change the dose or interrupt treatment with one of the medications.
Inform your doctor or pharmacist if you are using or have recently used other medications, even those purchased without a prescription.
Pregnancy and Breastfeeding
Consult your doctor or pharmacist before using any medication.
In case of pregnancy or breastfeeding, the medical team will decide on the convenience of using this medication.
3. How to use Cardi-Braun Maintenance
This medication will always be administered by specialized medical personnel.
Your cardiac surgeon and medical team will decide on the most suitable dose for you.
This medication is injected directly into the heart (intracardiac perfusion) through a device called a heart-lung machine or pulmonary bypass.
This device assumes the functions of the heart and is responsible for pumping and circulating blood. This allows your heart to remain still during the intervention and allows surgeons to operate on your heart without it moving or being filled with blood.
If you have been administered more Cardi-Braun Maintenance than you should:
It is unlikely that this will happen because your doctor will determine the most suitable dose for you.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Phone (91) 562 04 20 indicating the product and the amount administered.
If you have any other doubts about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, Cardi-Braun Maintenance can cause side effects, although not all people suffer from them.
Your surgeon will explain what possible side effects may arise from the use of Cardi-Braun Maintenance during the open-heart surgery you will undergo.
The following side effects of unknown frequency have been reported:
Cardiac disorders:
Myocardial infarction, ventricular arrhythmia, ventricular fibrillation.
Complementary tests:
Abnormal electrocardiogram, abnormal acid-base balance.
Metabolic and nutritional disorders:
Electrolyte imbalance, hyperglycemia (in patients receiving solutions with high glucose content; may require insulin administration).
Once the operation is finished and blood circulation is restored, the heartbeat may be delayed, so defibrillation of the heart (use of electric current to restore the heartbeat) and the use of medications to restore normal heart function may be necessary.
If you consider that any of the side effects you are suffering from is serious or if you notice any side effect not mentioned in this leaflet, inform your doctor or pharmacist.
Reporting of side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Cardi-Braun Maintenance
Keep out of the reach and sight of children.
No special storage conditions are required.
Do not use Cardi-Braun Maintenance after the expiration date indicated on the packaging. The expiration date is the last day of the month indicated.
Do not use Cardi-Braun Maintenance if the solution is cloudy or sedimented.
Do not use Cardi-Braun Maintenance if the packaging shows visible signs of deterioration.
Any unused solution must be disposed of in accordance with local requirements.
Medications should not be thrown away in drains or in the trash. Ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Additional information
Composition of Cardi-Braun Maintenance
The active ingredients are:
Per 1 mlPer 500 ml
Tromethamine 8.7560 mg 4.3780g
Sodium citrate 1.5840 mg 0.7920g
Citric acid monohydrate 0.1976 mg 0.0988g
Sodium dihydrogen phosphate dihydrate 0.1512 mg 0.0756g
Potassium chloride 1.7960 mg 0.8980g
Sodium chloride 9.8600 mg 4.9300g
Glucose monohydrate 34.6600 mg 17.3300 g
Electrolyte composition:
Phosphates 0.97 mmol/l
Citrate 6.32 mmol/l
Sodium 185.8 mmol/l
Potassium 24.1 mmol/l
Chloride 192.8 mmol/l
Acetate 23 mmol/l
The other components are: Acetic acid (pH adjustment) and water for injectable preparations
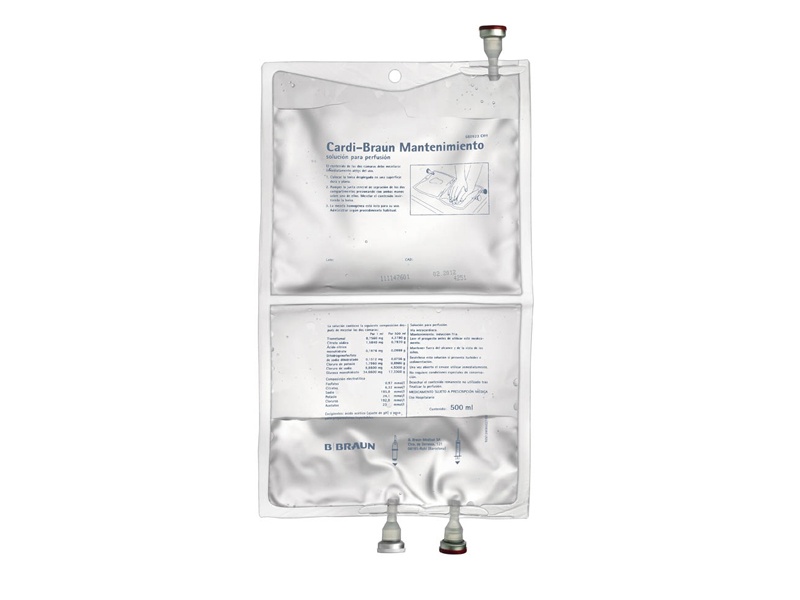
Appearance of the product and packaging content
Cardi-Braun Maintenance is a clear and transparent perfusion solution that comes in a single-unit packaging containing a 500 ml flexible plastic bag.
Marketing authorization holder and manufacturer
- Braun Medical, SA
Ctra. de Terrassa, 121
08191-Rubí (Barcelona)
This leaflet was approved in: December 2024
This information is intended only for doctors or healthcare professionals:
Cardi-Braun Maintenance solution for intracardiac perfusion is a ready-to-use solution.
Its administration is carried out through commercial systems that distribute the solution through the cardiopulmonary bypass device.
The volumes of solution that are perfused depend on the duration or type of open-heart surgical procedure.
The cardioplegic solutions Cardi-Braun can be administered to the coronary circulation through two routes - anterograde and retrograde - or in a mixed manner.
Cardi-Braun Maintenance requires the addition of potassium chloride 2M for the cold induction phase.The addition of the potassium chloride solution must be carried out under strict aseptic conditions
Instructions for the correct use of the cardioplegic solutions Cardi-Braun
Unfold the bag and place it on a hard surface. Open the central joint by pressing with both hands on one of the bag's chambers.

Mix the contents by inverting the bag several times. The homogeneous mixture is ready for use.

The addition of the potassium chloride solution can be incorporated directly into the bag through a syringe in the connector located at the bottom of the bag, using an aseptic procedure.

Remove the white cap from the perfusion connector. Clean the connector and insert the intracardiac perfusion equipment. Perfuse according to protocol.

Once the packaging is opened, use immediately.
Discard the unused fraction of the solution.
B|BRAUN
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CARDI-BRAUN MAINTENANCE SOLUTION FOR INFUSIONDosage form: INJECTABLE INFUSION, 500 mlActive substance: cardioplegia solutionsManufacturer: B Braun Medical S.A.Prescription requiredDosage form: NULL, -Active substance: cardioplegia solutionsManufacturer: Mit Gesundheit GmbhPrescription requiredDosage form: NULL, -Active substance: cardioplegia solutionsManufacturer: S.A.L.F. S.P.A. Laboratorio FarmacologicoPrescription required
Online doctors for CARDI-BRAUN MAINTENANCE SOLUTION FOR INFUSION
Discuss questions about CARDI-BRAUN MAINTENANCE SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















