CABAZITAXEL VIVANTA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION

How to use CABAZITAXEL VIVANTA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cabazitaxel Vivanta 60 mg concentrate and solvent for solution for infusion EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience side effects, ask your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
C
- What is Cabazitaxel Vivanta and what is it used for
- What you need to know before you are given Cabazitaxel Vivanta
- How to use Cabazitaxel Vivanta
- Possible side effects
- Storage of Cabazitaxel Vivanta
- Contents of the pack and other information
1. What is Cabazitaxel Vivanta and what is it used for
The name of your medicine is Cabazitaxel Vivanta. Its common name is cabazitaxel. It belongs to a group of medicines called "taxanes", used to treat cancers.
Cabazitaxel is used to treat prostate cancer that has progressed after receiving other chemotherapy. It works by stopping the growth of cells and their multiplication.
As part of your treatment, you will also take a corticosteroid (prednisone or prednisolone) every day by mouth. Ask your doctor about this other medicine.
2. What you need to know before you are given Cabazitaxel Vivanta
Do not use Cabazitaxel Vivanta
- if you are allergic (hypersensitive) to cabazitaxel, other taxanes, polysorbate 80, or any of the other ingredients of this medicine (listed in section 6),
- if your white blood cell count is very low (neutrophil count less than or equal to 1,500/mm3),
- if you have severe liver problems,
- if you have recently been or are going to be vaccinated against yellow fever.
You should not receive Cabazitaxel Vivanta if any of the above applies to you. If you are not sure, consult your doctor before receiving Cabazitaxel Vivanta.
Warnings and precautions
Before starting treatment with Cabazitaxel Vivanta, you will have blood tests to check that you have enough blood cells and that your kidneys and liver are working properly to receive cabazitaxel.
Tell your doctor immediately if:
- you have a fever. During treatment with cabazitaxel, it is more likely that your white blood cell count will decrease. Your doctor will monitor your blood and general condition to detect signs of infection. You may be given other medicines to keep your blood cell count up. People with low cell counts can develop life-threatening infections. The first sign of infection may be a fever, so if you have a fever, tell your doctor immediately.
- you have ever had any allergy. During treatment with cabazitaxel, severe allergic reactions can occur.
- you have severe or persistent diarrhea, feel unwell (nausea) or are sick (vomiting). Any of these situations can cause severe dehydration. Your doctor should give you treatment.
- you have a feeling of numbness, tingling, burning, or decreased sensations in your hands and feet.
- you have any bleeding problems in the intestine or have changes in the color of your stools or stomach pain. If the bleeding or pain is severe, your doctor will stop your treatment with cabazitaxel. This is because cabazitaxel may increase the risk of bleeding or development of perforations in the intestinal wall.
- you have kidney problems.
- you have yellowing of the skin and eyes, dark urine, severe nausea (feeling unwell) or vomiting, as these may be signs or symptoms of liver problems.
- you notice that the volume of your urine increases or decreases significantly.
- you have blood in your urine.
If any of the above applies to you, tell your doctor immediately. Your doctor may reduce the dose of this medicine or stop treatment.
Other medicines and Cabazitaxel Vivanta
Tell your doctor, pharmacist, or nurse if you are using or have recently used other medicines, including those obtained without a prescription. This is because some medicines can affect the effectiveness of this medicine or this medicine can affect the effectiveness of other medicines. These medicines include:
- ketoconazole, rifampicin (for infections);
- carbamazepine, phenobarbital, or phenytoin (for seizures);
- St. John's Wort or Hypericum (a medicinal plant used to treat depression and other problems);
- statins (such as simvastatin, lovastatin, atorvastatin, rosuvastatin, or pravastatin) (to reduce cholesterol in your blood);
- valsartan (for high blood pressure);
- repaglinide (for diabetes).
While you are being treated with this medicine, consult your doctor before getting vaccinated.
Pregnancy, breastfeeding, and fertility
This medicine is not intended for use in women.
Use condoms during sexual intercourse if your partner is or may become pregnant. Cabazitaxel may be present in your semen and can affect the fetus. It is recommended not to father a child during and up to 4 months after treatment and to ask for information about sperm preservation before treatment, as this medicine may alter male fertility.
Driving and using machines
During treatment with this medicine, you may feel tired or dizzy. If this happens, do not drive or use tools or machines until you feel better.
Cabazitaxel Vivanta contains ethanol (alcohol)
This medicine contains 573 mg of alcohol (ethanol) in each vial of solvent. The amount in the dose of this medicine is equivalent to less than 11 ml of beer or 5 ml of wine. The small amount of alcohol in this medicine does not produce any noticeable effect. If you are addicted to alcohol, have liver disease, or have epilepsy, consult your doctor or pharmacist before taking this medicine.
Cabazitaxel Vivanta contains polysorbate 80
Polysorbates can affect circulation and the heart (e.g., low blood pressure, changes in heartbeats).
3. How to use Cabazitaxel Vivanta
Before receiving cabazitaxel, you will be given anti-allergic medicines to reduce the risk of allergic reactions.
- This medicine will be administered by a doctor or nurse.
- This medicine must be prepared (diluted) before administration. This leaflet provides practical information for handling and administration of this medicine for doctors, nurses, and pharmacists.
- This medicine will be administered in the hospital through a drip (infusion) in one of your veins (intravenously) for approximately 1 hour.
- As part of your treatment, you will also take a corticosteroid medicine (prednisone or prednisolone) by mouth every day.
C
- The usual dose depends on your body surface area. Your doctor will calculate your body surface area in square meters (m2) and decide the dose you should receive.
- Usually, you will receive an infusion every 3 weeks.
If you have any further questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, cabazitaxel can cause side effects, although not everybody gets them. Your doctor will discuss this with you and explain the risks and potential benefits of your treatment.
Go to your doctor immediately if you notice any of the following side effects:
- fever (high temperature). This is common (may affect up to 1 in 10 people).
- severe loss of body fluids (dehydration). This is common (may affect up to 1 in 10 people). This can happen if you have severe or persistent diarrhea, or fever, or if you have been vomiting.
- severe stomach pain or stomach pain that does not go away. This can happen if you have a perforation in the stomach, esophagus, or intestine (gastrointestinal perforation). This can cause death.
If any of the above applies to you, tell your doctor immediately.
Other side effects include:
Very common(may affect more than 1 in 10 people):
- reduction in the number of red blood cells (anemia), or white blood cells (which are important for fighting infections)
- reduction in the number of platelets (which results in an increased risk of bleeding)
- loss of appetite (anorexia)
- stomach upset, including nausea, vomiting, diarrhea, or constipation
- back pain
- blood in the urine
- fatigue, weakness, or lack of energy.
Common(may affect up to 1 in 10 people):
- altered taste
- shortness of breath
- cough
- abdominal pain
- short-term hair loss (in most cases, hair grows back normally)
- joint pain
- urinary tract infection
- low white blood cell count associated with fever and infection
- feeling of numbness, tingling, burning, or decreased sensations in hands and feet
- dizziness
- headache
- increased or decreased blood pressure
- stomach upset, heartburn, or belching
- stomach pain
- hemorrhoids
- muscle spasms
- frequent or painful urination
- urinary incontinence
- kidney problems or changes
- mouth or lip ulcers
- infections or risk of infections
- high blood sugar levels
- insomnia
- mental confusion
- feeling of anxiety
- feeling of numbness or loss of sensation or pain in hands and feet
- balance problems
- rapid or irregular heartbeats
- blood clots in the legs or lungs
- feeling of suffocation in the skin
- mouth or throat pain
- rectal bleeding
- muscle disorders, weakness, or pain
- swelling of feet or legs
- chills
- nail disorders (change in nail color; nails may come off).
Uncommon(may affect up to 1 in 100 people):
- low potassium levels in the blood
- ringing in the ears
- feeling of heat in the skin
- red skin
- bladder inflammation, which can occur when your bladder has previously been exposed to radiation therapy (radiation recall cystitis).
Frequency not known(cannot be estimated from the available data)
- interstitial lung disease (inflammation of the lungs causing cough and difficulty breathing).
Reporting of side effects:
If you experience any side effects, tell your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Surveillance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cabazitaxel Vivanta
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the vials after EXP. The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions.
In the section "Practical information for healthcare professionals on the preparation, administration, and handling of Cabazitaxel Vivanta 60 mg concentrate and solvent for solution for infusion" includes information on the storage and shelf-life of Cabazitaxel Vivanta, once it has been diluted and is ready for use.
The disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Cabazitaxel Vivanta
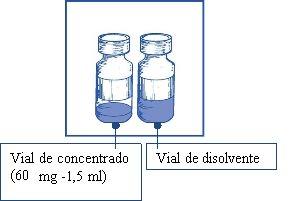
The active substance is cabazitaxel. 1 ml of concentrate contains 40 mg of cabazitaxel. One vial of concentrate contains 60 mg of cabazitaxel.
The other ingredients are polysorbate 80 and citric acid in the concentrate, and ethanol 96% and water for injections in the solvent (see section 2 "Cabazitaxel Vivanta contains ethanol (alcohol)").
Note: Both the vial of Cabazitaxel Vivanta 60 mg/1.5 ml concentrate (fill volume: 73.2 mg of cabazitaxel/1.83 ml) and the vial of solvent (fill volume: 5.67 ml) contain an overfill to compensate for liquid loss during preparation. This overfill ensures that after dilution with the complete contents of the provided solvent, there is a solution containing 10 mg/ml of cabazitaxel.
Appearance of the product and pack contents
Cabazitaxel Vivanta is a concentrate and solvent for solution for infusion (sterile concentrate).
The concentrate is a clear, colorless to pale yellow viscous solution.
The solvent is a clear, colorless solution.
A pack of Cabazitaxel Vivanta contains:
- A single-use glass vial, closed with a chlorobutyl rubber stopper, sealed with an aluminum cap, covered with a flip-off plastic cap, containing 1.5 ml (nominal volume) of concentrate.
- A single-use glass vial, closed with a chlorobutyl rubber stopper, sealed with an aluminum cap, covered with a flip-off plastic cap, containing 4.5 ml (nominal volume) of solvent.
Marketing authorization holder
Vivanta Generics s.r.o.
Trtinová 26001, Cakovice
196 00 Prague 9
Czech Republic
Manufacturer
Pharmadox Healthcare Ltd,
KW20A Kordin Industrial Park,
Paola, PLA3000,
Malta
You can obtain more information about this medicine by contacting the local representative of the marketing authorization holder:
Local Representative:
Vivanta Generics s.r.o. branch in Spain
C/Guzmán el Bueno, 133, edificio Britannia
28003 Madrid
This medicine is authorized in the Member States of the European Economic Area under the following names:
Netherlands Cabazitaxel Vivanta 60 mg concentraat en oplosmiddel voor oplossing voor infusie
Ireland Cabazitaxel MSN
Spain Cabazitaxel Vivanta 60 mg concentrado y disolvente para solución para perfusión EFG
Germany Cabazitaxel AXiromed 60 mg Konzentrat und Lösung zur Herstellung einer Infusionslösung
Poland Cabazitaxel Medical Valley
Finland Cabazitaxel Medical Valley 60 mg infuusiokonsentraatti ja liuotin, liuosta varten
Denmark Cabazitaxel Medical Valley
Sweden Cabazitaxel Medical Valley 60 mg koncentrat och vätska till infusionsvätska, lösning
Norway Cabazitaxel Medical Valley
Date of last revision of this leaflet:September 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
The following information is intended for healthcare professionals only.
PRACTICAL INFORMATION FOR HEALTHCARE PROFESSIONALS ON THE PREPARATION, ADMINISTRATION, AND HANDLING OF CABAZITAXEL VIVANTA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION
This information complements sections 3 and 5 for the user.
It is important that you read the entire contents of this procedure before preparing the infusion solution.
Incompatibilities
This medicine must not be mixed with other medicines except those used for dilutions.
Shelf-life and special precautions for storage
For the pack of Cabazitaxel Vivanta 60 mg concentrate and solvent
After opening the vial
The vials of concentrate and solvent must be used immediately. If they are not used immediately, the time and storage conditions are the responsibility of the user. From a microbiological point of view, the two-stage dilution process should be carried out under controlled and aseptic conditions (see "Preparation and administration precautions" below).
After initial dilutionof Cabazitaxel Vivanta 60 mg concentrate with the completecontents of the solvent vial: chemical and physical stability has been demonstrated during use for 1 hour at room temperature.
After final dilution in the infusion bag/bottle
The chemical and physical stability of the infusion solution has been demonstrated for 8 hours at room temperature (15°C - 30°C) including 1 hour of infusion time and for 48 hours in the refrigerator including infusion time.
From a microbiological point of view, the infusion solution should be used immediately. If it is not used immediately, the storage times and conditions are the responsibility of the user and should normally not exceed 24 hours at 2°C - 8°C, unless the dilution has been carried out under controlled and validated aseptic conditions.
Precautions for preparation and administration
As with other antineoplastic agents, caution should be exercised during the preparation and administration of Cabazitaxel Vivanta solutions, taking into account the use of safety devices, personal protective equipment (e.g., gloves), and preparation procedures.
If at any stage of preparation, Cabazitaxel Vivanta comes into contact with the skin, wash immediately and thoroughly with water and soap. If it comes into contact with mucous membranes, wash immediately and thoroughly with water.
Cabazitaxel Vivanta should only be prepared and administered by personnel trained in the handling of cytotoxic agents. Pregnant workers should not handle it.
Always dilute the concentrate for infusion solution with the completesolvent provided before adding it to the infusion solutions.
Preparation stages
Read this entire section carefully before mixing and diluting. Cabazitaxel Vivanta requires TWOdilutions before administration. Follow the preparation instructions provided below.
Note: Both the vial of Cabazitaxel Vivanta 60 mg/1.5 ml concentrate (fill volume: 73.2 mg of cabazitaxel/1.83 ml) and the vial of solvent (fill volume: 5.67 ml) contain an overfill to compensate for liquid loss during preparation. This overfill ensures that after dilution with the COMPLETEsolvent provided, a solution containing 10 mg/ml of cabazitaxel is obtained.
To prepare the infusion solution, the following two-stage dilution process must be carried out aseptically.
Stage 1: Initial dilution of the concentrate for infusion solution with the provided solvent.
Stage 1.1
Inspect the vial of concentrate and the provided solvent. The concentrate and solvent solutions should be transparent and practically free of particles.
 Stage 1.2
Stage 1.2
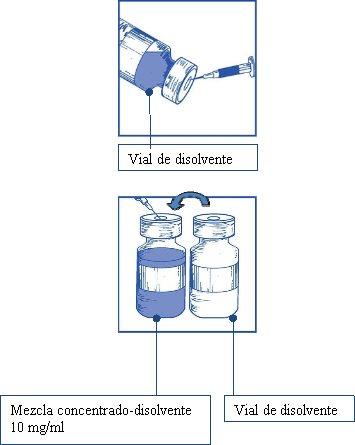
Using a syringe with a fixed needle, aseptically withdraw the completecontents of the provided solvent by partially inverting the vial.
Stage 1.3
Inject the completecontents into the corresponding vial of concentrate.
To limit foam formation as much as possible when injecting the solvent, direct the needle towards the inner wall of the concentrate vial and inject slowly.
Once reconstituted, the resulting solution contains 10 mg/ml of cabazitaxel.
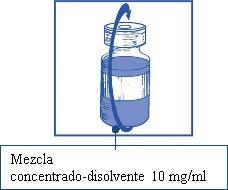
Stage 1.4
Remove the syringe and needle, and manually mix gently by repeated inversions until a transparent and homogeneous solution is obtained. This may take about 45 seconds.
Stage 1.5
Allow the solution to stand for approximately 5 minutes, then check that the solution is homogeneous and transparent.
It is normal for foam to persist after this time.
This resulting concentrate-solvent mixture contains 10 mg/ml of cabazitaxel (at least 6 ml of released volume). The second dilution should be performed immediately (before 1 hour) as detailed in Stage 2.
More than one vial of concentrate-solvent mixture may be necessary to administer the prescribed dose.
Stage 2: Final dilution (for infusion)
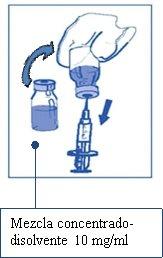
Stage 2.1
Aseptically withdraw the required amount of concentrate-solvent mixture (10 mg/ml of cabazitaxel) using a graduated syringe with a fixed needle. For example, a dose of 45 mg of Cabazitaxel Vivanta would require 4.5 ml of the concentrate-solvent mixture prepared in Stage 1.
As foam may still be present on the wall of the vial of this solution after the preparation described in Stage 1, it is preferable to position the syringe needle in the middle of the contents during withdrawal.
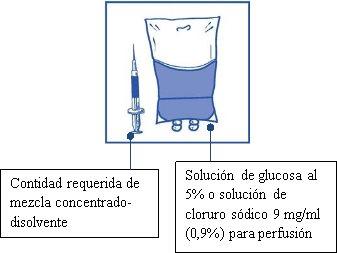
Stage 2.2
Inject into a sterile, non-PVC container of 5% glucose solution or 0.9% sodium chloride solution for infusion. The concentration of the infusion solution should be between 0.10 mg/ml and 0.26 mg/ml.

Stage 2.3
Remove the syringe and mix the contents of the infusion bag or bottle manually by rocking.
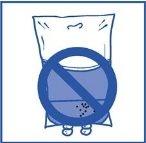
Stage 2.4
As with all parenteral products, the resulting infusion solution should be visually inspected before use. Since the infusion solution is supersaturated, it may crystallize over time. In this case, the solution should not be used and should be discarded.
The infusion solution should be used immediately. However, the in-use storage time may be longer under the specific conditions mentioned in the section Validity period and special storage precautions.
Disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
Method of administration
Cabazitaxel Vivanta is administered by infusion over 1 hour.
The use of a 0.22-micron nominal pore size filter (also known as 0.2 microns) is recommended during administration.
PVC infusion containers or polyurethane infusion sets should not be used for the preparation and administration of the infusion solution.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CABAZITAXEL VIVANTA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 20 mg/mlActive substance: cabazitaxelManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE INFUSION, 20 mgActive substance: cabazitaxelManufacturer: Eugia Pharma (Malta) LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 60 mgActive substance: cabazitaxelManufacturer: Reddy Pharma Iberia S.A.Prescription required
Online doctors for CABAZITAXEL VIVANTA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION
Discuss questions about CABAZITAXEL VIVANTA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions