
BUCOPRO SPRAY 8.75 mg/dose ORAL SPRAY SOLUTION

How to use BUCOPRO SPRAY 8.75 mg/dose ORAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Bucopro Spray 8, 75 mg/dose Oral Spray Solution
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this package leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 3 days.
Contents of the Package Leaflet
- What is Bucopro Spray and what is it used for
- What you need to know before taking Bucopro Spray
- How to take Bucopro Spray
- Possible side effects
- Storage of Bucopro Spray
- Package Contents and Additional Information
1. What is Bucopro Spray and what is it used for
The active ingredient is flurbiprofen. Bucopro Spray belongs to a group of medications called non-steroidal anti-inflammatory drugs (NSAIDs), which work by modifying the body's response to pain, inflammation, and fever.
Bucopro Spray is used for the short-term relief of throat pain symptoms such as irritation, pain, difficulty swallowing, and inflammation in adults over 18 years of age.
2. What you need to know before taking Bucopro Spray
Do not use Bucopro Spray
- if you are allergic to flurbiprofen, other non-steroidal anti-inflammatory drugs (NSAIDs), acetylsalicylic acid, or any of the other components of this medication (listed in section 6).
- if you have ever had an allergic reaction after taking non-steroidal anti-inflammatory drugs (NSAIDs) or acetylsalicylic acid, e.g., asthma, wheezing, itching, nasal discharge, hives, swelling.
- if you have or have had two or more episodes of stomach or intestinal ulcers or bleeding.
- if you have had severe colitis (intestinal inflammation).
- if you have ever had blood clotting problems or bleeding problems after taking NSAIDs.
- if you are in the last trimester of pregnancy.
- if you have severe heart, kidney, or liver failure.
- if you are under 18 years of age.
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Bucopro Spray if:
- You are already taking another non-steroidal anti-inflammatory drug (NSAID) or acetylsalicylic acid.
- You have tonsillitis (inflammation of the tonsils) or think you may have contracted a bacterial infection in the throat (as you may need antibiotics).
- You are an elderly patient (as you are more likely to suffer from side effects).
- You have asthma or allergies.
- You have a skin disease called systemic lupus erythematosus or mixed connective tissue disease.
- You have high blood pressure.
- You have had an intestinal disease (ulcerative colitis, Crohn's disease).
- You have heart, kidney, or liver problems.
- You have had a stroke.
- If you are in the first 6 months of pregnancy or are breastfeeding.
- You have an infection - see the "Infections" section below.
During the use of Bucopro Spray:
- At the first sign of skin reaction (rash, peeling, blistering) or other sign of allergic reaction, stop using this medication and consult a doctor immediately.
- Inform your doctor of any unusual abdominal symptoms you experience (especially bleeding).
- If it does not improve, worsens, or new symptoms appear, consult your doctor.
- Medications like flurbiprofen may be associated with a slight increase in the risk of myocardial infarction or stroke. Any risk is greater when the doses are high or the treatment is prolonged. Do not exceed the recommended dose or treatment duration (see section 3).
Infections
Non-steroidal anti-inflammatory drugs (NSAIDs) may hide signs of infections such as fever and pain. This can delay the start of appropriate treatment for the infection, which can lead to a greater risk of complications. If you take this medication while having an infection and your symptoms persist or worsen, consult your doctor or pharmacist without delay.
Children and Adolescents
This medication should not be used by children and adolescents under 18 years of age.
Using Bucopro Spray with other medications
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication, including those purchased without a prescription. In particular:
- Other non-steroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 inhibitors for pain and inflammation, as these may increase the risk of stomach or intestinal bleeding.
- Warfarin, acetylsalicylic acid, and other anticoagulant medications.
- ACE inhibitors, angiotensin II antagonists (medications that lower blood pressure).
- Diuretics, including potassium-sparing diuretics.
- Selective serotonin reuptake inhibitors (SSRIs) for depression.
- Digitalis (for heart problems) such as digoxin.
- Cyclosporin (to prevent organ rejection after a transplant).
- Corticosteroids (to reduce inflammation).
- Lithium (for mood disorders).
- Methotrexate (for psoriasis, arthritis, and cancer).
- Mifepristone (used to induce abortion); NSAIDs should not be used in the 8-12 days following mifepristone administration because they may reduce its effect.
- Oral antidiabetics.
- Phenytoin (for epilepsy).
- Probenecid and sulfinpyrazone (for gout and arthritis).
- Quinolone antibiotics (for bacterial infections) such as ciprofloxacin or levofloxacin.
- Tacrolimus (immunosuppressant used after organ transplantation).
- Zidovudine (for HIV infection).
Using Bucopro Spray with food and drinks
Do not consume alcohol during treatment with this medication, as it may increase the risk of bleeding in the stomach or intestine.
Pregnancy, Breastfeeding, and Fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Oral forms (e.g., tablets) of flurbiprofen may cause adverse effects in unborn babies. It is unknown if there is the same risk with Bucopro Spray.
Pregnancy
Do not use this medication if you are in the last trimester of pregnancy.
Do not take it during the first six months of pregnancy unless it is clearly necessary and advised by your doctor. If you need treatment during this period, the lowest possible dose should be used for the shortest possible time.
Breastfeeding
Do not use this medication if you are breastfeeding.
Fertility
Flurbiprofen belongs to a group of medications that may affect female fertility. This effect is reversible when the medication is stopped. It is unlikely that occasional use of this medication can affect your chances of becoming pregnant; however, inform your doctor before using this medication if you have problems becoming pregnant.
Driving and Using Machines
This medication should not affect your ability to drive or use machines. However, dizziness, drowsiness, and visual disturbances are possible side effects of NSAIDs. If you experience these effects, do not drive or use machines.
Bucopro Spray contains ethanol
This medication contains a small amount of ethanol (alcohol), less than 100 mg per dose (3 sprays).
3. How to use Bucopro Spray
Follow the administration instructions for this medication indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is:
Adults from 18 years:apply a dose of 3 sprays administered to the back of the throat every 3-6 hours as needed, up to a maximum of 5 doses in a 24-hour period.
A dose (3 sprays) contains 8.75 mg of flurbiprofen.
Use in Children and Adolescents
This medication should not be administered to children and adolescents under 18 years of age.
For oral use only.
- Spray only to the back of the throat.
- Do not inhale while spraying.
- Do not apply more than 5 doses (15 sprays) in 24 hours.
Bucopro Spray is for short-term use only.
Use the lowest effective dose for the shortest necessary time to relieve symptoms. If you have an infection, consult your doctor or pharmacist without delay if symptoms (such as fever and pain) persist or worsen (see section 2). In case of oral irritation, treatment with flurbiprofen should be suspended.
Do not use this medication for more than 3 days unless advised by your doctor.
If it does not improve, worsens, or new symptoms appear, consult your doctor or pharmacist.
Preparing the Spray Pump
When you are going to use the spray pump for the first time (or after the medication has been stored for a long time), you must shake the container and prepare the spray pump.
Point the nozzle away from you and perform a minimum of 4 sprays until a fine, homogeneous spray appears. The spray pump is now ready for use. If you have not used the medication for some time, point the nozzle away from you and perform at least 1 spray to ensure a fine, homogeneous spray is produced. Before administering the medication, always ensure a fine, homogeneous spray is generated.
Performing the Spray
Hold the bottle upright with the nozzle directed towards the back of the throat.
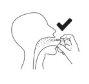
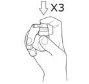
Press the spray pump 3times, with a quick and continuous movement, making sure to press it all the way down for each spray, and remove your finger from the top of the pump between each spray.
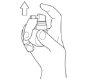
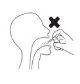
Do not inhale while spraying.
If you use more Bucopro Spray than you should
In case of overdose or accidental ingestion, consult a doctor or pharmacist immediately or call the Toxicology Information Service, telephone 91 562 04 20, indicating the medication and the amount ingested.
The symptoms of an overdose may be: nausea, vomiting, stomach pain, or, more rarely, diarrhea. You may also experience ringing in the ears, headache, and gastrointestinal bleeding.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone gets them.
STOP USINGthis medication and consult a doctor immediately if you experience:
- Signs of allergic reaction, such as asthma, wheezing, or difficulty breathing without apparent cause, itching, nasal discharge, or hives.
- Swelling of the face, tongue, or throat that causes difficulty breathing, palpitations, and a drop in blood pressure that leads to cardiocirculatory shock (all these effects can occur even with the first use of this medication).
- Signs of hypersensitivity and skin reactions such as redness, swelling, peeling, blistering, scaling, or ulcers on the skin and mucous membranes.
Other side effects may occur:
Tell your doctor or pharmacist if you notice any of the following side effects, or any other effect not described in this package leaflet:
Common(may affect up to 1 in 10 people)
- dizziness, headache
- throat irritation
- mouth ulcers, pain or numbness in the mouth
- throat pain
- discomfort (feeling of heat or burning or tingling) in the mouth
- nausea and diarrhea
- prickling and itching of the skin
Uncommon(may affect up to 1 in 100 people)
- drowsiness
- blistering in the mouth or throat, numbness of the throat
- abdominal swelling and pain, gas, constipation, indigestion, vomiting
- dry mouth
- burning sensation in the mouth and alteration of taste
- rash and itching on the skin
- fever, pain
- feeling of numbness or difficulty sleeping
- worsening of asthma, wheezing, difficulty breathing
- reduced sensitivity in the throat
Rare(may affect up to 1 in 1,000 people)
- anaphylactic reaction
Frequency not known(cannot be estimated from the available data)
- anemia, thrombocytopenia (low platelet count in the blood, which can lead to bruising and bleeding)
- swelling (edema), high blood pressure, heart failure, or myocardial infarction
- severe skin reactions, including blistering reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis (rare diseases due to severe adverse reactions to medications or infections in which there is a severe reaction of the skin or mucous membranes)
- hepatitis (inflammation of the liver)
Reporting Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use (www.notificaram.es). By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Bucopro Spray
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date that appears on the bottle and blister. The expiration date (CAD) is the last day of the month indicated.
Do not refrigerate or freeze.
Do not use this medication for more than 1 month after its first use.
Medications should not be disposed of through wastewater or household waste. Deposit the containers and medications you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Bucopro Spray:
The active ingredient is flurbiprofen. A dose (3 sprays) contains 8.75 mg of flurbiprofen. A spray contains 2.91 mg of flurbiprofen. One milliliter of oral spray solution contains 17.16 mg of flurbiprofen.
The other components are:
Betadex (E459)
Hydroxypropyl betadex
Disodium phosphate dodecahydrate
Citric acid
Sodium hydroxide
Cherry flavor
Sodium saccharin (E954)
Purified water
Qualitative composition of cherry flavor:
Flavoring substance(s)
Flavoring preparation(s)
Ethanol
Glycerol triacetate (E1518)
Propylene glycol (E1520)
Ascorbic acid (E300)
DL-α-tocopherol (E307)
Water
Appearance of the Product and Package Contents
Bucopro Spray 8.75 mg oral spray solution is a clear, colorless solution with a cherry flavor and odor.
This medication is presented in a plastic bottle with the solution contained in a dispenser equipped with a mechanical spray pump.
Each bottle contains 15 ml of solution that provides 88 sprays.
Marketing Authorization Holder
Laboratorios Cinfa, S.A.
Carretera Olaz-Chipi, 10. Polígono Industrial Areta
31620 Huarte (Navarra) – Spain
Manufacturer
Laboratorium Sanitatis
P.T. Alava
Calle Leonardo Da Vinci, 11
01510 Miñano (Álava)
Spain
Laboratorios Cinfa, S.A.
Carretera Olaz-Chipi, 10. Polígono Industrial Areta
31620 Huarte (Navarra) – Spain
This medication is authorized in EEA member states under the following names:
Names:
Portugal: Mentocaína Spray
Date of the last revision of this package leaflet:October 2023
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BUCOPRO SPRAY 8.75 mg/dose ORAL SPRAY SOLUTIONDosage form: BUCCAL/SUCKING TABLET, 8.75 mgActive substance: flurbiprofenManufacturer: Alfasigma España, S.L.Prescription not requiredDosage form: BUCCAL/SUCKING TABLET, 8.75 mgActive substance: flurbiprofenManufacturer: Alfasigma España, S.L.Prescription not requiredDosage form: BUCCAL/SUCKING TABLET, 8.75 mgActive substance: flurbiprofenManufacturer: Laboratorios Cinfa S.A.Prescription not required
Online doctors for BUCOPRO SPRAY 8.75 mg/dose ORAL SPRAY SOLUTION
Discuss questions about BUCOPRO SPRAY 8.75 mg/dose ORAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








