ATOMOXETINE TECNIGEN 4 mg/ml ORAL SOLUTION

How to use ATOMOXETINE TECNIGEN 4 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Atomoxetine TecniGen 4 mg/ml Oral Solution EFG
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Atomoxetine TecniGen and what is it used for
- What you need to know before you take Atomoxetine TecniGen
- How to take Atomoxetine TecniGen
- Possible side effects
- Storing Atomoxetine TecniGen
- Contents of the pack and other information
1. What is Atomoxetine TecniGen and what is it used for
What is it used for
This medicine contains atomoxetine and is used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in:
- children over 6 years of age
- adolescents
- adults
It is used as part of a comprehensive treatment programme, which includes psychological, educational, and social measures.
It is not intended for use in children under 6 years of age, as the safety and efficacy in this age group have not been established.
In adults, Atomoxetine TecniGen is used to treat ADHD when the symptoms are very troublesome and affect work or social life and when symptoms of the disorder were present during childhood.
How it works
Atomoxetine TecniGen increases the amount of noradrenaline in the brain. This is a naturally occurring substance that increases attention and decreases impulsiveness and hyperactivity in patients with ADHD. This medicine has been prescribed to help control the symptoms of ADHD. This medicine is not a stimulant and does not cause dependence.
It may take a few weeks before you start to feel better. Be patient and talk to your doctor if you have any questions or concerns.
About ADHD
Children and adolescents with ADHD find it:
- hard to sit still
- hard to concentrate
This is not because they are not trying, many children and adolescents without ADHD may have similar problems. However, with ADHD, these problems occur more frequently and can be more severe, making everyday life more difficult. Children and adolescents with ADHD may have difficulty learning and doing homework. They may find it hard to behave well at home, in school, or in other places. ADHD does not affect intelligence.
Adults with ADHD find it hard to do all the things that children with ADHD find hard to do. This may mean that they have problems with:
- work
- relationships
- low self-esteem
- education
2. What you need to know before you take Atomoxetine TecniGen
Do not take Atomoxetine TecniGen if you:
- are allergic to atomoxetine or any of the other ingredients of this medicine (listed in section 6)
- have taken a medicine called a monoamine oxidase inhibitor (MAOI), such as phenelzine, within the last two weeks. MAOIs are sometimes used for depression and other mental health conditions; taking atomoxetine with an MAOI could cause a serious reaction
- have an eye problem called narrow-angle glaucoma
- have a serious heart problem that may get worse if you take atomoxetine
- have serious blood vessel problems in your brain, such as a stroke, or problems with the blood vessels in your brain, such as an aneurysm or vascular stenosis
- have a tumour of your adrenal gland (phaeochromocytoma)
Do not take atomoxetine if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before taking this medicine, as atomoxetine may make these problems worse.
Warnings and precautions
Both adults and children should be aware of the following warnings and precautions. Talk to your doctor or pharmacist before taking atomoxetine if you:
- have thoughts of suicide or have tried to harm yourself
- have a heart problem or an increased heart rate
- have high blood pressure
- have low blood pressure
- have problems with sudden changes in blood pressure or heart rate
- have a cardiovascular disease or have had a stroke
- have liver problems; you may need a lower dose
- have psychotic symptoms, such as hearing voices or seeing things that are not there
- have mania (feelings of elation or over-excitement)
- have agitation
- have aggressive feelings
- have hostility
- have a history of epilepsy or have had seizures for any other reason; atomoxetine may increase the risk of seizures
- have a mood disorder, such as depression
- have repeated movements that you cannot control
Talk to your doctor or pharmacist if you have any of the above symptoms before starting treatment, as atomoxetine may make these problems worse. Your doctor will want to monitor how the medicine affects you.
Aggressive behaviour, hostility, or emotional instability
Treatment with Atomoxetine TecniGen may make you feel aggressive, hostile, or violent, or make these symptoms worse if they were already present before treatment. It may also cause changes in your behaviour or mood, such as physical aggression, threatening behaviour, and thoughts of harming others. If you or your family and/or friends notice any of these reactions, talk to your doctor or pharmacist immediately.
Serotonin syndrome
Serotonin syndrome is a potentially life-threatening condition that may occur when taking Atomoxetine TecniGen with other medicines (see section 2, "Other medicines and Atomoxetine TecniGen"). The signs and symptoms of serotonin syndrome can include a combination of the following: confusion, restlessness, lack of coordination, and stiffness, hallucinations, coma, rapid heartbeat, increased body temperature, changes in blood pressure, sweating, flushing, tremors, hyperactive reflexes, nausea, vomiting, and diarrhoea. Contact a doctor or go to the nearest hospital emergency department immediately if you think you are experiencing serotonin syndrome.
Tests your doctor will do before you start taking Atomoxetine
These tests are to decide if atomoxetine is the right medicine for you.
Your doctor will:
- check your blood pressure and heart rate (pulse) before and while you are taking atomoxetine
- check your weight and height if you are a child or adolescent while you are taking atomoxetine
Your doctor will ask you about:
- other medicines you are taking
- if you have a family history of sudden death
- any other medical problems you or your family may have
It is important that you provide all the information you can. This will help your doctor decide if atomoxetine is the right medicine for you. Your doctor may decide to do other medical tests before starting treatment with this medicine.
Other medicines and Atomoxetine TecniGen
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. This includes medicines that you buy without a prescription. Your doctor will decide if you can take atomoxetine with other medicines and may need to adjust the dose or increase it more slowly.
Do not take atomoxetine with MAOIs (monoamine oxidase inhibitors) taken for depression. See section 2, "Do not take Atomoxetine TecniGen if you:"
If you are taking other medicines, atomoxetine may affect how they work or cause side effects. If you are taking any of the following medicines, check with your doctor or pharmacist before taking atomoxetine:
- medicines that increase blood pressure or are used to control it
- medicines such as antidepressants, for example imipramine, venlafaxine, mirtazapine, fluoxetine, and paroxetine
- some cough and cold remedies that contain medicines that can affect blood pressure; when buying these products, it is important to check with your pharmacist
- some medicines used to treat mental health conditions
- medicines that are known to increase the risk of seizures
- some medicines that make atomoxetine stay in the body longer than usual (such as quinidine and terbinafine)
- salbutamol (a medicine for asthma) when taken by mouth or injected, may make you feel like your heart is racing, but this will not make your asthma worse
The following medicines may increase the risk of an abnormal heart rhythm when taken with atomoxetine:
- medicines used to control heart rhythm
- medicines that alter the levels of salts in the blood
- medicines for the prevention and treatment of malaria
- antibiotics (such as erythromycin and moxifloxacin)
Atomoxetine TecniGen may affect or be affected by other medicines. These include:
- Some antidepressants, opioids such as tramadol, and medicines used to treat migraine called triptans. These medicines may interact with Atomoxetine TecniGen and cause serotonin syndrome, a potentially life-threatening condition (see section 2, Warnings and precautions, Serotonin syndrome)
If you are not sure if the medicines you are taking are listed above, talk to your doctor or pharmacist before taking atomoxetine.
Pregnancy and breast-feeding
It is not known if this medicine can affect the unborn baby or pass into breast milk.
This medicine should not be taken during pregnancy unless your doctor advises you to do so.
You should avoid taking this medicine if you are breast-feeding.
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Atomoxetine may make you feel tired or sleepy. Be careful when driving or using machinery until you know how atomoxetine affects you. If you feel tired or sleepy, do not drive or use machinery.
Important information about the oral solution
This oral solution may irritate your eyes. If the oral solution comes into contact with your eyes, rinse them immediately with plenty of water and talk to your doctor. If your hands or any other part of your body come into contact with the oral solution, wash the area with water as soon as possible.
Atomoxetine TecniGen contains sorbitol
This medicine contains 47.10 mg of sorbitol in each ml.
Sorbitol is a source of fructose. If your doctor has told you that you (or your child) have an intolerance to some sugars, or have been diagnosed with a rare genetic disorder called hereditary fructose intolerance (HFI), talk to your doctor before taking this medicine.
Atomoxetine TecniGen contains sodium benzoate
This medicine contains 0.80 mg of sodium benzoate in each ml.
Atomoxetine TecniGen contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per ml; this is essentially "sodium-free".
Atomoxetine TecniGen contains propylene glycol
This medicine contains 9.44 mg of propylene glycol in each ml.
Atomoxetine TecniGen contains ethanol
This medicine contains 0.0025% ethanol (alcohol), which is equivalent to 0.02 mg/ml.
3. How to take Atomoxetine TecniGen
Follow the instructions for administration of this medicine given by your doctor or pharmacist. If you are not sure, talk to your doctor or pharmacist. It is usually taken once or twice a day (in the morning and late afternoon or early evening).
- Children should not take this medicine without the help of an adult.
- If you feel sleepy or nauseous when taking atomoxetine once a day, your doctor may change the dosing to twice a day.
- The medicine can be taken with or without food.
- The oral solution should not be mixed with food or water, as this may reduce the amount taken or make the taste less pleasant.
- Taking the medicine at the same time each day can help you remember to take it.
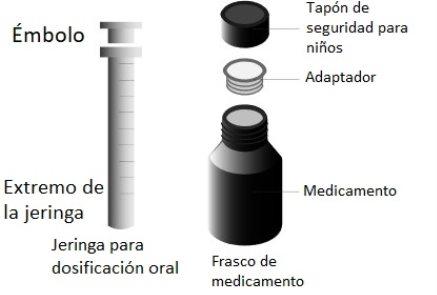
Atomoxetine oral solution is available in a bottle. This forms part of a pack that also includes a dosing device, which consists of a 10 ml oral syringe graduated in 1 ml fractions and a bottle adapter. To see the instructions on how to use the adapter and the dosing syringe, read the user manual instructions included in the pack.
How much to take
If you are a child (over 6 years of age) or an adolescent:
Your doctor will tell you how much atomoxetine to take, based on your weight. You will usually start with a low dose before increasing it, based on your weight.
- Up to 70 kg body weight: starting with a total daily dose of 0.5 mg per kg body weight for a minimum of 7 days. Your doctor will then decide if the dose should be increased to the usual maintenance dose of 1.2 mg per kg body weight per day.
- Over 70 kg body weight: starting with a total daily dose of 40 mg for a minimum of 7 days. Your doctor will then decide if the dose should be increased to the usual maintenance dose of 80 mg per day. The maximum daily dose is 100 mg.
Adults
Atomoxetine should be started with a total daily dose of 40 mg for a minimum of 7 days. Your doctor will then decide if the dose should be increased to the usual maintenance dose of 80 mg to 100 mg per day. The maximum daily dose is 100 mg.
If you have liver problems, your doctor may recommend a lower dose.
If you take more Atomoxetine TecniGen than you should
In case of overdose or accidental ingestion, talk to your doctor or pharmacist immediately or call the Toxicology Information Service, telephone: 91 562 04 20, stating the medicine and the amount taken. The most commonly reported symptoms after an overdose are gastrointestinal symptoms, sleepiness, dizziness, tremors, and abnormal behaviour. Very rarely, serotonin syndrome, a potentially life-threatening condition, has also been reported (see section 2, Warnings and precautions, Serotonin syndrome).
If you forget to take Atomoxetine TecniGen
If you miss a dose, take another as soon as you remember, but do not take more than the total daily dose in a 24-hour period. Do not take a double dose to make up for a forgotten dose.
If you stop taking Atomoxetine TecniGen
If you stop taking atomoxetine, you will not normally get any withdrawal effects, but the symptoms of ADHD may return. You should talk to your doctor before stopping treatment.
What your doctor will do while you are taking Atomoxetine TecniGen
Your doctor will do some tests:
- before you start - to make sure atomoxetine is safe and suitable for you
- after you start - at least every 6 months, although this may be more often
These tests will also be done when the dose is changed. The tests will include:
- measuring height and weight in children and adolescents
- measuring blood pressure and heart rate
- checking for any problems or if side effects have got worse while you are taking atomoxetine
Long-term treatment
Atomoxetine does not need to be taken indefinitely. If you take atomoxetine for more than a year, your doctor will review your treatment to see if the medicine is still needed.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. Although some people suffer from adverse effects, most people consider that atomoxetine helps them. Your doctor will discuss these adverse effects with you.
Some adverse effects could be serious. If you experience any of the following effects, contact your doctor immediately.
Uncommon(may affect up to 1 in 100 people)
- feeling or having a very fast heart rate, abnormal heart rhythm
- thoughts or feelings of suicide
- feeling aggressive
- feeling hostile or angry
- mood swings
- severe allergic reaction with symptoms of
- swelling of the face and throat
- difficulty breathing
- hives (small, itchy, red rashes on the skin)
- seizures
- psychotic symptoms including hallucinations (such as hearing voices or seeing things that do not exist), believing things that are not true, or being suspicious.
Children and young people under 18 years have a higher risk of suffering from adverse effects such as:
- thoughts or feelings of suicide (may affect up to 1 in 100 people)
- mood swings (may affect up to 1 in 10 people)
Adults have a lower risk(may affect up to 1 in 1,000 people) of suffering from adverse effects such as:
- seizures
- psychotic symptoms including hallucinations (such as hearing voices or seeing things that do not exist), believing things that are not true, or being suspicious.
Rare(may affect up to 1 in 1,000 people)
- liver problems
Stop treatment with atomoxetine and contact your doctor immediately if you experience any of the following adverse effects:
- dark urine
- yellowing of the skin and eyes
- pain when pressing on the upper right side of the abdomen, just below the ribs
- unexplained feeling of nausea
- fatigue
- itching
- feeling of starting with a cold
Other reported adverse effects have been the following. If any of them worsen, consult your doctor or pharmacist.
Adverse effects very common(may affect more than 1 in 10 people) | |
CHILDREN over 6 years and YOUNG PEOPLE | ADULTS |
(pulse) These effects may disappear over time in most patients |
|
Adverse effects common(may affect up to 1 in 10 people) | |
CHILDREN over 6 years and YOUNG PEOPLE | ADULTS |
|
|
Adverse effects uncommon(may affect up to 1 in 100 people) | |
CHILDREN over 6 years and YOUNG PEOPLE | ADULTS |
burning, pinching, itching, or tingling
|
QT interval)
|
Adverse effects rare(may affect up to 1 in 1,000 people) | |
CHILDREN over 6 years and YOUNG PEOPLE | ADULTS |
|
|
Adverse effects of unknown frequency | |
CHILDREN over 6 years and YOUNG PEOPLE | ADULTS |
|
Effects on Growth
When some children start taking atomoxetine, their growth (weight and height) is reduced. However, with long-term treatment, children recover the weight and height appropriate for their age group.
Your doctor will monitor your child's height and weight. If your child is not growing or gaining weight as expected, your doctor may change the dose or temporarily suspend treatment with atomoxetine.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Medication Monitoring System for Human Use: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Atomoxetina TecniGen
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging and on the bottle after CAD. The expiration date is the last day of the month indicated.
Do not use the oral solution more than 45 days after the first opening of the bottle.
This medicine does not require special storage conditions.
Medicines should not be thrown away through the sewers or in the trash. Deposit the packaging and medicines that you no longer need in the SIGRE point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines that you no longer need. This way, you will help protect the environment.
6. Container Content and Additional Information
Composition of Atomoxetina TecniGen
- The active ingredient is atomoxetine hydrochloride. Each ml of oral solution contains 4 mg of atomoxetine (as hydrochloride).
- The other components are: Liquid Sorbitol (E420), Xylitol (E697), Sucralose, Sodium Benzoate (E211), Sodium Dihydrogen Phosphate Dihydrate, Strawberry Flavor (containing Propylene Glycol and Ethanol), Phosphoric Acid, Sodium Hydroxide, and Purified Water.
Appearance of the Product and Container Content
Oral solution, 4 mg/ml (clear, colorless)
Atomoxetina oral solution 4 mg/ml oral solution EFG is available in a 125 ml amber glass bottle with a child-resistant cap. The packaging also includes a dosing device consisting of a 10 ml oral syringe graduated in 1 ml fractions and a bottle adapter.
Atomoxetina oral solution 4 mg/ml oral solution EFG is available in a package containing a single bottle and in a multiple package with three bottles.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Tecnimede España Industria Farmacéutica, S.A.
Avda. de Bruselas, 13, 3º D. Edificio América. Polígono Arroyo de la Vega,
28108 Alcobendas (Madrid)
Spain
Manufacturer
Atlantic Pharma – Produções Farmacêuticas, S.A.
Rua da Tapada Grande, n.º 2
Abrunheira, 2710-089 Sintra
Portugal
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Portugal - Atomoxetina Pentafarma
Spain – Atomoxetina TecniGen 4 mg/ml oral solution EFG
Date of the last revision of this leaflet: October 2024
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
Instructions for Use
Your step-by-step guide to using Atomoxetina TecniGen
WHEN USING Atomoxetina TecniGen, READ AND FOLLOW THE STEP-BY-STEP INSTRUCTIONS CAREFULLY.
WARNING:The adapter poses a CHOKING HAZARD due to its small size. Do not attach the syringe to the adapter until the adapter is fully inserted into the bottle. For safe use, the adapter must be fully inserted into the bottle. Use only under adult supervision.
IMPORTANT
DO NOTlet your child take the medication without your help.
DO NOTuse the medication if it has expired (check the expiration date on the label).
DO NOTuse the medication if more than 45 days have passed since you first opened the bottle. See the Disposalsection for more information on what to do with unused medication.
DO NOTwash the oral syringe with soap or detergent. DO NOTput the syringe in the dishwasher. If you do, the syringe may not work as well as it should. Please see the cleaning instructions from step N to P.
It is not recommended to mix Atomoxetina TecniGen oral solution with food or water because it may affect the taste or prevent the full dose from being taken.
Atomoxetina TecniGen can cause eye irritation. AVOID EYE CONTACT. If the medication comes into contact with the eyes, rinse them immediately with water and consult a doctor. Wash your hands and any surfaces that may have come into contact with the medication as soon as possible.
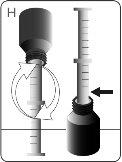
STEP 1 INITIAL BOTTLE PREPARATION
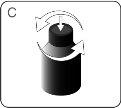
| Before the first use, push the adapter until it is fully seatedon the bottle. Do not assemble the syringe onto the adapter until the adapter is fully inserted into the bottle. DO NOTturn the adapter. WARNING:The adapter poses a CHOKING HAZARD due to its small size. For safe use, it must be fully inserted into the bottle. |
STEP 2 PREPARATION
| Gather all the items you will need:
|
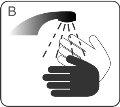
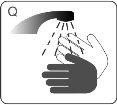
| Wash your hands with water and soap |
Write your child's dose here:
________ ml
Make sure it is the exact dose prescribed by your child's doctor.
IF YOUR CHILD'S DOSE IS 10 ml OR LESS, you will use the syringe 1 time
IF YOUR CHILD'S DOSE IS MORE THAN 10 mlbut NOT more than 20 ml, you will use the same syringe 2 times.
IF YOUR CHILD'S DOSE IS MORE THAN 20 ml, you will use the same syringe 3 times.
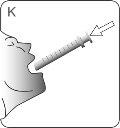
STEP 3 OBTAINING THE DOSE
| Press the screw cap downwards while turning it counterclockwise. Remove the screw cap from the bottle. |
| Push the plunger completely to the end of the syringe. |
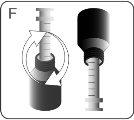
| Insert the tip of the syringe into the adapter. Make sure the tip of the syringe is fully seated in the adapter. |
| Turn the bottle and syringe upside down. |
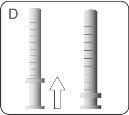
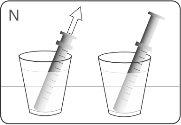
| Hold the bottle and syringe with 1 hand. With the other hand, pull the plunger downwards to draw the appropriate amount of medication into the syringe. Check that the dose in the syringe is the correct one. Turn the bottle upside down and place it on a flat surface. Check that there are no air bubbles in the syringe. If there are, return the medication to the bottle and repeat steps D to H. An air bubble can result in an incorrect dose. |
| Remove the syringe from the bottle. DO NOTtouch the plunger. |
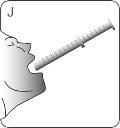
STEP 4 ADMINISTERING THE MEDICATION
| Place the tip of the syringe on one side of your child's mouth. Tell your child not to bite the syringe. |
| Push the plunger downwards until ALLthe medication is in your child's mouth. DO NOTdirect the medication straight into your child's throat. Make sure your child swallows all the medication. |
| IF YOUR CHILD'S DOSE IS MORE THAN 10 ml, repeat from step D to K to give the remaining dose. Use the same syringe. See the dosing chartin this document. Make sure you administer the exact dose prescribed by your child's doctor. |
STEP 5 CLEANING
| Tighten the bottle cap firmly. DO NOTremove the adapter. The cap will adapt. |
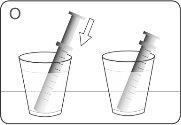
| Fill a glass with clean water. DO NOTwash the oral syringe with soap or detergent. DO NOTremove the plunger from the oral syringe. Place the tip of the syringe in the water. Pull the plunger upwards to fill it with water. |
| Push the plunger downwards and empty the water into the glass or sink. |
| Remove excess water from the syringe. Dry the syringe with a paper towel. |
| Wash your hands and your child's hands with soap and water. DO NOTtouch your eyes after coming into contact with Atomoxetina TecniGen. Atomoxetina TecniGen can irritate your eyes. |
Consult the rest of the document to find answers to frequently asked questions, check the dosing chart, and other important information.
DOSING CHART FOR DOSES OF MORE THAN 10 ml
Use this chart to calculate the dose to be administered to your child. Find the correct dose in the first column. Consult with your doctor or pharmacist on how to administer the correct dose to your child.
If this is your child's dose | Put this amount of Atomoxetina TecniGen in the syringe, for the first time | Put this amount of Atomoxetina TecniGen in the syringe, a second time | Put this amount of Atomoxetina TecniGen in the syringe, a third time |
11 ml | 10 ml | 1 ml | 0 |
12 ml | 10 ml | 2 ml | 0 |
13 ml | 10 ml | 3 ml | 0 |
14 ml | 10 ml | 4 ml | 0 |
15 ml | 10 ml | 5 ml | 0 |
16 ml | 10 ml | 6 ml | 0 |
17 ml | 10 ml | 7 ml | 0 |
18 ml | 10 ml | 8 ml | 0 |
19 ml | 10 ml | 9 ml | 0 |
20 ml | 10 ml | 10 ml | 0 |
21 ml | 10 ml | 10 ml | 1 ml |
22 ml | 10 ml | 10 ml | 2 ml |
23 ml | 10 ml | 10 ml | 3 ml |
24 ml | 10 ml | 10 ml | 4 ml |
25 ml | 10 ml | 10 ml | 5 ml |
Frequently Asked Questions
- What happens if there is an air bubble in the oral syringe?
- DO NOTadminister the medication to your child. Bubbles can make the dose incorrect. Empty the medication back into the bottle and repeat from step D to H.
- What happens if there is too much medication in the syringe?
- Hold the tip of the syringe in the bottle. Hold the bottle upside down. Push the plunger downwards until the correct dose is in the syringe.
- What happens if there is not enough medication in the syringe?
R. Hold the tip of the syringe in the bottle. Hold the bottle upside down. Pull the plunger downwards until the correct dose is in the syringe.
- What do I do if the medication comes into contact with my eyes or my child's eyes?
R. Rinse your eyes immediately with water and consult a doctor. As soon as possible, wash your hands and any surfaces that may have come into contact with the medication.
- How do I travel with this medication?
R. Make sure you have enough Atomoxetina TecniGen for the entire trip. Keep the medication in a safe place at room temperature.
- Can I mix Atomoxetina TecniGen with food or water before giving it to my child?
R. It is not recommended to mix Atomoxetina TecniGen oral solution with food or water. It may affect the taste of the oral solution or prevent the full dose from being taken. Your child can drink a glass of water after taking the entire dose of the medication.
Storage
This medicinal product does not require special storage conditions.
Keep the bottle and syringe out of the sight and reach of children.
Disposal
Do not throw away medicines directly down the drain or into the trash. Consult your pharmacist on how to dispose of unused medicines. These measures will help protect the environment.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ATOMOXETINE TECNIGEN 4 mg/ml ORAL SOLUTIONDosage form: CAPSULE, 10 mgActive substance: atomoxetineManufacturer: Laboratorios Rubio S.A.Prescription requiredDosage form: CAPSULE, 100 mgActive substance: atomoxetineManufacturer: Laboratorios Rubio S.A.Prescription requiredDosage form: CAPSULE, 18 mgActive substance: atomoxetineManufacturer: Laboratorios Rubio S.A.Prescription required
Online doctors for ATOMOXETINE TECNIGEN 4 mg/ml ORAL SOLUTION
Discuss questions about ATOMOXETINE TECNIGEN 4 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions