AQUIPTA 10 mg TABLETS

How to use AQUIPTA 10 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
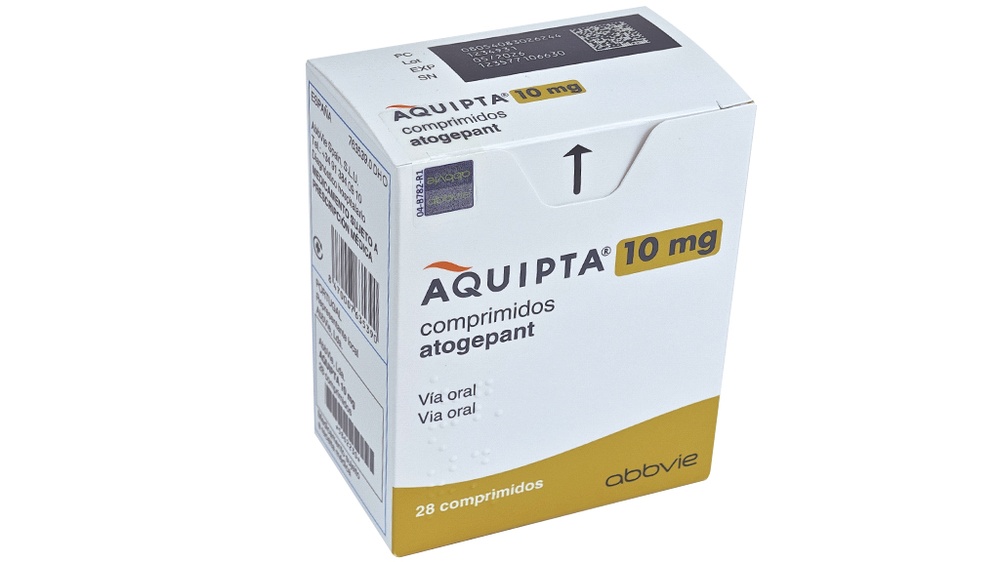
AQUIPTA 10 mg tablets
AQUIPTA 60 mg tablets
atogepant
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is AQUIPTA and what is it used for
- What you need to know before you take AQUIPTA
- How to take AQUIPTA
- Possible side effects
- Storage of AQUIPTA
- Contents of the pack and other information
1. What is AQUIPTA and what is it used for
AQUIPTA contains the active substance atogepant. AQUIPTA is used to prevent migraine in adults who have at least 4 days of migraine per month.
AQUIPTA is thought to block the activity of the calcitonin gene-related peptide (CGRP) receptor family, which have been linked to migraine.
2. What you need to know before you take AQUIPTA
Do not take AQUIPTA
- if you are allergic to atogepant or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Stop taking AQUIPTA and tell your doctor immediately if you experience any symptoms of an allergic reaction such as:
- difficulty breathing
- swelling of the face
- rash, itching or hives
Some of these symptoms may occur within 24 hours after the first use. Sometimes they may occur several days after taking AQUIPTA.
Tell your doctor, pharmacist or nurse before you start taking AQUIPTA if you have severe liver problems.
Children and adolescents
Do not give this medicine to children or adolescents under 18 years of age, as the use of AQUIPTA in this age group has not been studied.
Other medicines and AQUIPTA
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Some medicines may increase the risk of side effects (see section 4).
The following list contains examples of medicines that may require your doctor to reduce the dose of AQUIPTA:
- ketoconazole, itraconazole, clarithromycin, rifampicin (medicines used to treat fungal or bacterial infections);
- ritonavir (medicine used to treat HIV);
- cyclosporin (medicine that affects your immune system).
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
If you are pregnant, you should not take AQUIPTA. If you are a woman who could become pregnant, you should use adequate contraceptive methods during treatment with AQUIPTA.
If you are breastfeeding or planning to breastfeed, you should not take AQUIPTA. You and your doctor will decide whether to breastfeed or take AQUIPTA.
Driving and using machines
AQUIPTA may make you feel sleepy. Do not drive or use machines if you are affected.
AQUIPTA contains sodium
AQUIPTA 10 mg tablets
This medicine contains less than 1 mmol of sodium (23 mg) per tablet; this is essentially “sodium-free”.
AQUIPTA 60 mg tablets
This medicine contains 31.5 mg of sodium (main component of cooking/table salt) per tablet. This is equivalent to 1.6% of the maximum recommended daily intake of sodium for an adult.
3. How to take AQUIPTA
Take this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
How much to take
The recommended dose is 60 mg of atogepant once a day. Your doctor may tell you to take a lower dose if:
- you are taking other medicines (mentioned in section 2);
- you have severe kidney problems or are on dialysis.
How to take
AQUIPTA is taken by mouth. Do not break, crush, chew or split the tablet before swallowing. The tablets can be taken with or without food.
If you take more AQUIPTA than you should
If you take more tablets than you should, talk to your doctor. You may experience some of the side effects mentioned in section 4.
If you forget to take AQUIPTA
- If you forget a dose, take it as soon as you remember.
- If you forget during the whole day, skip the missed dose and take a dose as usual the next day.
- Do not take a double dose to make up for forgotten doses.
If you stop taking AQUIPTA
Do not stop taking AQUIPTA without talking to your doctor first. Symptoms may come back if you stop treatment.
If you have any other questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Stop taking AQUIPTA and contact your doctor immediately if you have any of the following symptoms, which may be part of a severe allergic reaction:
- difficulty breathing
- swelling of the face
- rash, itching or hives
Other side effects
Tell your doctor if you notice any of the following side effects:
Common(may affect up to 1 in 10 people):
- nausea (feeling sick);
- constipation;
- fatigue (tiredness);
- sleepiness (drowsiness);
- decreased appetite;
- weight loss.
Uncommon(may affect up to 1 in 100 people):
- increased levels of liver enzymes.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of AQUIPTA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What AQUIPTA contains
AQUIPTA 10 mg tablets
- The active substance is atogepant. Each tablet contains 10 mg of atogepant.
- The other ingredients are: copolymer of polyvinylpyrrolidone/vinyl acetate, polyethylene glycol succinate and vitamin E, mannitol, microcrystalline cellulose, sodium chloride, sodium croscarmellose, colloidal silicon dioxide and sodium stearyl fumarate (see section 2).
AQUIPTA 60 mg tablets
- The active substance is atogepant. Each tablet contains 60 mg of atogepant.
- The other ingredients are: copolymer of polyvinylpyrrolidone/vinyl acetate, polyethylene glycol succinate and vitamin E, mannitol, microcrystalline cellulose, sodium chloride, sodium croscarmellose, colloidal silicon dioxide and sodium stearyl fumarate (see section 2).
Appearance and packaging
AQUIPTA 10 mg tablets
The 10 mg tablet of AQUIPTA is a biconvex, round, white to off-white tablet with the inscription “A” and “10” on one side. It is available in packs containing 28 or 98 tablets.
AQUIPTA 60 mg tablets
The 60 mg tablet of AQUIPTA is a biconvex, oval, white to off-white tablet with the inscription “A60” on one side. It is available in packs containing 28 or 98 tablets.
Not all pack sizes may be marketed.
Marketing authorisation holder
AbbVie Deutschland GmbH & Co. KG
Knollstrasse
67061 Ludwigshafen
Germany
Manufacturer
AbbVie S.r.l
S.R. 148 Pontina Km 52 Snc
Campoverde di Aprilia, Latina 04011
Italy
You can request more information about this medicine from the local representative of the marketing authorisation holder:
België/Belgique/Belgien AbbVie SA Tél/Tel: +32 10 477811 | Lietuva AbbVie UAB Tel: +370 5 205 3023 |
| Luxembourg/Luxemburg AbbVie SA Belgique/Belgien Tél/Tel: +32 10 477811 |
Ceská republika AbbVie s.r.o. Tel: +420 233 098 111 | Magyarország AbbVie Kft. Tel.:+36 1 455 8600 |
Danmark AbbVie A/S Tlf: +45 72 30-20-28 | Malta Vivian Corporation Ltd. Tel: +356 27780331 |
Deutschland AbbVie Deutschland GmbH & Co. KG Tel: 00800 222843 33 (gebührenfrei) Tel: +49 (0) 611 / 1720-0 | Nederland AbbVie B.V. Tel: +31 (0)88 322 2843 |
Eesti AbbVie OÜ Tel: +372 623 1011 | Norge AbbVie AS Tlf: +47 67 81 80 00 |
Ελλάδα AbbVie ΦΑΡΜΑΚΕΥΤΙΚΗ Α.Ε. Τηλ: +30 214 4165 555 | Österreich AbbVie GmbH Tel: +43 1 20589-0 |
España AbbVie Spain, S.L.U. Tel: +34 91 384 09 10 | Polska AbbVie Sp. z o.o. Tel.: +48 22 372 78 00 |
France AbbVie Tél: +33 (0) 1 45 60 13 00 | Portugal AbbVie, Lda. Tel: +351 (0)21 1908400 |
Hrvatska AbbVie d.o.o. Tel + 385 (0)1 5625 501 | România AbbVie S.R.L. Tel: +40 21 529 30 35 |
Ireland AbbVie Limited Tel: +353 (0)1 4287900 | Slovenija AbbVie Biofarmacevtska družba d.o.o. Tel: +386 (1)32 08 060 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika AbbVie s.r.o. Tel: +421 2 5050 0777 |
Italia AbbVie S.r.l. Tel: +39 06 928921 | Suomi/Finland AbbVie Oy Puh/Tel: +358 (0)10 2411 200 |
Κύπρος Lifepharma (Z.A.M.) Ltd Τηλ.: +357 22 34 74 40 | Sverige AbbVie AB Tel: +46 (0)8 684 44 600 |
Latvija AbbVie SIA Tel: +371 67605000 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu/.
To listen to or request a copy of this leaflet in
, contact the local representative of the marketing authorisation holder.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AQUIPTA 10 mg TABLETSDosage form: TABLET, 60 mgActive substance: atogepantManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription requiredDosage form: INJECTABLE, 140 mgActive substance: erenumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 70 mgActive substance: erenumabManufacturer: Novartis Europharm LimitedPrescription required
Online doctors for AQUIPTA 10 mg TABLETS
Discuss questions about AQUIPTA 10 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions