
AIMOVIG 140 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN

How to use AIMOVIG 140 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Aimovig 70 mg solution for injection in pre-filled pen
Aimovig 140 mg solution for injection in pre-filled pen
erenumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Aimovig and what is it used for
- What you need to know before you use Aimovig
- How to use Aimovig
- Possible side effects
- Storing Aimovig
- Contents of the pack and other information
1. What is Aimovig and what is it used for
Aimovig contains the active substance erenumab. It belongs to a group of medicines called monoclonal antibodies.
Aimovig works by blocking the activity of the CGRP molecule, which has been linked to migraine (CGRP stands for calcitonin gene-related peptide).
Aimovig is used to prevent migraine in adults who have at least 4 days of migraine per month at the start of treatment with Aimovig.
2. What you need to know before you use Aimovig
Do not use Aimovig
- if you are allergic to erenumab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before you start using Aimovig:
- if you have ever had an allergic reaction to latex. The container of this medicine contains latex rubber in the cap.
- if you have a cardiovascular disease. Aimovig has not been studied in patients with certain cardiovascular diseases.
Talk to your doctor or seek immediate medical attention:
- if you have any symptoms of a severe allergic reaction, such as rash or swelling, normally of the face, mouth, tongue, or throat; or difficulty breathing. Severe allergic reactions can occur within minutes of using Aimovig, but some may occur more than a week later.
- contact your doctor if you have constipation and seek immediate medical attention if you develop constipation with severe abdominal pain or vomiting, abdominal swelling, or bloating. Constipation may occur with treatment with Aimovig. It is usually mild or moderate. However, some patients who have used Aimovig have had constipation with serious complications and have been hospitalized. Some cases have required surgical intervention.
Children and adolescents
This medicine must not be used in children and adolescents (under 18 years) as it has not been studied in this age group.
Other medicines and Aimovig
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
Pregnancy
Your doctor will decide whether you should stop using Aimovig during pregnancy.
Breastfeeding
Monoclonal antibodies like Aimovig are known to pass into breast milk during the first few days after birth, but after this initial period, Aimovig can be used. Talk to your doctor about using Aimovig during breastfeeding to help you decide whether to stop breastfeeding or stop using Aimovig.
Driving and using machines
Aimovig is unlikely to affect your ability to drive or use machines.
Aimovig contains sodium
Aimovig contains less than 1 mmol of sodium (23 mg) per dose; this is essentially “sodium-free”.
3. How to use Aimovig
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, talk to your doctor again.
If your doctor prescribes the 70 mg dose, you should have one injection every 4 weeks. If your doctor prescribes the 140 mg dose, you should have one injection of Aimovig 140 mg or two consecutive injections of Aimovig 70 mg, every 4 weeks. If two injections of Aimovig 70 mg are given, the second injection should be given immediately after the first, in a different injection site. Make sure to inject the entire contents of both pens.
Aimovig is given as an injection under the skin (i.e., a subcutaneous injection). You or your caregiver can give the injection in the abdomen or thigh. The outer aspect of the upper arm can also be used as an injection site, but only if someone else is giving you the injection. If you need 2 injections, they should be given in different sites to avoid the skin becoming hardened and should not be given in areas where the skin is sensitive, damaged, red, or hardened.
Your doctor or nurse will teach you or your caregiver the correct way to prepare and inject Aimovig. Do not try to inject Aimovig until you have received this training.
If you have not noticed any effect of the treatment after 3 months, inform your doctor, who will decide whether you should continue treatment.
The Aimovig pens are for single use only.
You can find detailed instructions on how to administer Aimovig in the section “Instructions for use of Aimovig pre-filled pen” at the end of this leaflet.
If you use more Aimovig than you should
If you have received more Aimovig than you should, or if the dose has been given before it was due, talk to your doctor.
If you forget to use Aimovig
- If you forget a dose of Aimovig, you should give it as soon as possible after you remember.
- Then contact your doctor, who will tell you when to give the next dose. Follow the new schedule exactly as your doctor tells you.
If you stop using Aimovig
Do not stop using Aimovig without talking to your doctor first. If you stop using Aimovig, your symptoms may come back.
If you have any other questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may happen with this medicine. Most of these effects are mild to moderate.
Common: may affect up to 1 in 10 people
- allergic reactions such as rash, swelling, hives, or difficulty breathing (see section 2)
- constipation
- itching
- muscle spasms
- injection site reactions, such as pain, redness, and swelling at the injection site.
Frequency not known (cannot be estimated from the available data)
- skin reactions such as rash, itching, hair loss, or ulcers in the mouth or lip.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Aimovig
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label after EXP and on the carton after CAD. The expiry date is the last day of the month shown.
Store the pen(s) in the outer carton to protect from light. Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Once Aimovig has been removed from the refrigerator, it should be stored at room temperature (up to 25°C) in the outer carton and used within 7 days, or otherwise discarded. Once Aimovig has been removed from the refrigerator, it must not be put back.
Do not use this medicine if you notice that the solution contains particles, is cloudy, or has a clearly yellow color.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
Aimovig Composition
- The active ingredient is erenumab.
- Aimovig 70 mg injectable solution in a pre-filled pen contains 70 mg of erenumab.
- Aimovig 140 mg injectable solution in a pre-filled pen contains 140 mg of erenumab.
- The other components are sucrose, polysorbate 80, sodium hydroxide, glacial acetic acid, and water for injectable preparations.
Product Appearance and Container Contents
Aimovig injectable solution is transparent to opalescent, colorless to light yellow, and practically free of particles.
Aimovig is available in containers that contain a single-use pre-filled pen and in multiple containers that contain 3 (3x1) pre-filled pens.
Only some pack sizes may be marketed.
Marketing Authorization Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Sandoz GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Germany
Novartis Pharmaceutical Manufacturing GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lithuania SIA Novartis Baltics Lithuanian branch Tel: +370 5 269 16 50 |
Bulgaria Novartis Bulgaria EOOD Tel: +359 2 489 98 28 | Luxembourg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Czech Republic Novartis s.r.o. Tel: +420 225 775 111 | Hungary Novartis Hungária Kft. Tel: +36 1 457 65 00 |
Denmark Novartis Healthcare A/S Tel: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Germany Novartis Pharma GmbH Tel: +49 911 273 0 | Netherlands Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estonia SIA Novartis Baltics Estonian branch Tel: +372 66 30 810 | Norway Novartis Norge AS Tel: +47 23 05 20 00 |
Greece Novartis (Hellas) A.E.B.E. Tel: +30 210 281 17 12 | Austria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Spain Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Poland Novartis Poland Sp. z o.o. Tel: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tel: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croatia Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | Romania Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italy Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finland Novartis Finland Oy Tel: +358 (0)10 6133 200 |
Cyprus Novartis Pharma Services Inc. Tel: +357 22 690 690 | Sweden Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvia SIA Novartis Baltics Tel: +371 67 887 070 | United Kingdom (Northern Ireland) Novartis Ireland Limited Tel: +44 1276 698370 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu
Instructions for Use of Aimovig Pre-filled Pens
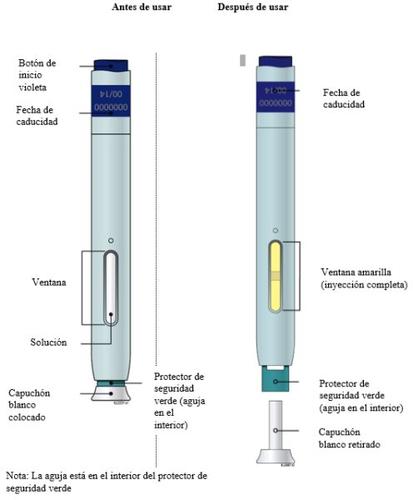
Aimovig 70 mg Pen Scheme(with light blue body, purple start button, white cap, and green safety protector)
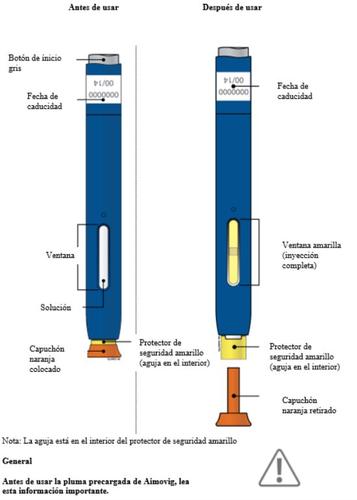
Aimovig 140 mg Pen Scheme(with dark blue body, gray start button, orange cap, and yellow safety protector)
Step 1: Preparation
Note: The dose of Aimovig that you have been prescribed is either 70 mg or 140 mg. This means that for the 70 mg dose, you need to inject the contents of one single-use 70 mg pen. For the 140 mg dose, you need to inject the contents of one single-use 140 mg pen or two single-use 70 mg pens consecutively.
(A)
Carefully remove the Aimovig pre-filled pen(s) from the box. Depending on the dose you have been prescribed, you may need to use one or two pens. Do not shake.
To avoid any discomfort at the injection site, keep the pen(s) at room temperature for at least 30 minutes before injection.
Note: Do not attempt to warm the pen(s) using a heat source such as hot water or a microwave.
(B)
Visually examine the pen(s). Make sure the solution you see through the window is transparent and colorless or light yellow.
Note:
- Do not use the pen(s) if any component appears cracked or broken.
- Do not use any pen that has been dropped.
- Do not use the pen if the cap is missing or not firmly attached.
In all the above cases, use a new pen, and if you have any doubts, contact your doctor or pharmacist.
(C)
Gather all the necessary materials for the injection(s). Wash your hands thoroughly with water and soap. On a clean and well-lit work surface, place:
|
|
(D)
Prepare and clean the injection site(s).
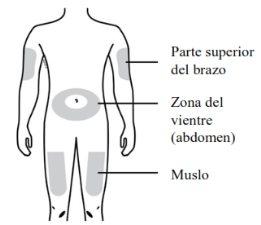
Use only the following injection sites:
- Thigh
- Abdomen (except for an area of 5 centimeters around the navel)
- Outer area of the upper arm (only if someone else is administering the injection)
Clean the injection site with an alcohol swab and let the skin dry.
Choose a different site each time you inject. If you need to use the same injection site, make sure it is not the same spot you used last time.
Note:
- After cleaning the site, do not touch it again before injection.
- Do not choose a site where the skin is sensitive, damaged, red, or hardened. Avoid injecting in areas with scars or stretch marks.
Step 2: Prepare
(E)
When you are ready for the injection (not before), remove the cap by pulling it straight off. From this moment on, the injection must be administered within 5 minutes. It is normal to see a drop of liquid at the tip of the needle or safety protector.
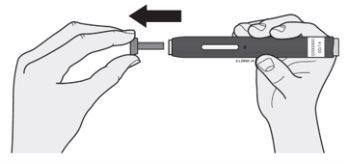
Note:
- Do not leave the cap off for more than 5 minutes, as the medication may dry out.
- Do not twist or bend the cap.
- Do not put the cap back on the pen after it has been removed.
- Do not put your fingers inside the safety protector.
(F)
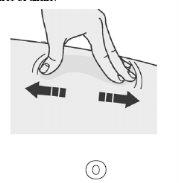
Prepare a firm surface at the chosen injection site (thigh, abdomen, or outer area of the upper arm) using eitherthe Stretch Method orthe Pinch Method.
Stretch Method
Stretch the skin firmly by moving your thumb and other fingers in opposite directions to define an area of about fivecentimeters wide.
Pinch Method
Pinch the skin firmly between your thumb and other fingers to define an area of about fivecentimeters wide.
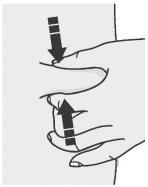
Note: It is essential to keep the skin stretched or pinched while injecting.
Step 3: Injection
(G)
Keep the skin stretched/pinched. After removing the cap, place the safety protector of the pen on the skin at a 90-degree angle. The needle is inside the safety protector.
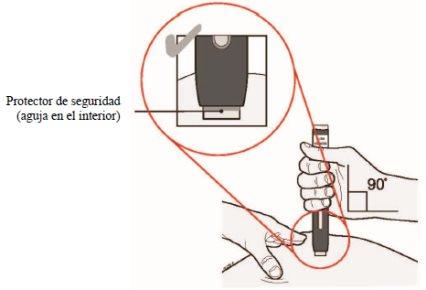
Note: Do not touch the start button yet.
(H)
Press the pen firmly against the skin until it no longer moves.
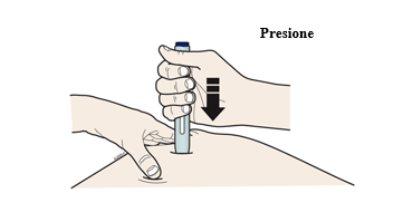
Note: You must press firmly but do not touch the start button until you are ready to inject.
(I)
Press the start button. You will hear a "click".
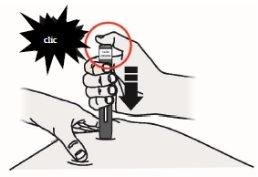
(J)
Lift your thumb off the button, but keep the pen pressed against the skin. The injection may take about 15 seconds.
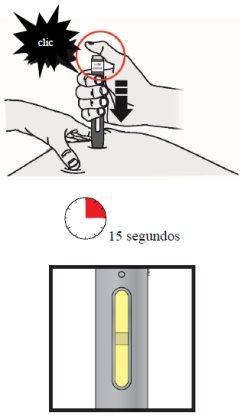
Note: When the injection is complete, the window will change from transparent to yellow, and you may hear a second "click".
Note:
- When you lift the pen off the skin, the needle will be automatically covered by the safety protector.
- If, when you remove the pen, the window does not turn yellow or if it appears that the medication is still being injected, this means you have not received a complete dose. Contact your doctor immediately.
Step 4: Finish
(K)
Discard the used pen and cap.
Place the used pen in a sharps disposal container immediately after use. Consult your doctor or pharmacist about the correct disposal method. There may be local regulations regarding this.
Note:
- Do not reuse the pen.
- Do not recycle the pen or the sharps disposal container.
- Always keep the sharps disposal container out of the reach of children.
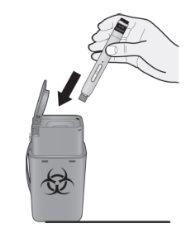
(L)
Examine the injection site.
If there is any blood on the skin, press a cotton ball or gauze over the injection site. Do not rub the injection site. If necessary, apply a bandage.
If you have been prescribed a dose of 140 mg and are using two Aimovig 70 mg pens, repeat the steps from 1(D) to 4 with the second pen to complete the dose. |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AIMOVIG 140 mg SOLUTION FOR INJECTION IN A PRE-FILLED PENDosage form: INJECTABLE, 70 mgActive substance: erenumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 225 mgActive substance: fremanezumabManufacturer: Teva GmbhPrescription requiredDosage form: INJECTABLE, 225 mgActive substance: fremanezumabManufacturer: Teva GmbhPrescription required
Online doctors for AIMOVIG 140 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Discuss questions about AIMOVIG 140 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions

















