ANDEMBRY 200 mg INJECTABLE SOLUTION IN PRE-FILLED PEN


How to use ANDEMBRY 200 mg INJECTABLE SOLUTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ANDEMBRY 200 mg solution for injection in pre-filled pen
garadacimab
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is ANDEMBRY and what is it used for
- What you need to know before you use ANDEMBRY
- How to use ANDEMBRY
- Possible side effects
- Storage of ANDEMBRY
- Contents of the pack and other information
- Instructions for use
1. What is ANDEMBRY and what is it used for
ANDEMBRY contains the active substance garadacimab.
ANDEMBRY is a medicine used in patients from 12 years of age with hereditary angioedema (HAE) to prevent attacks of angioedema.
HAE is a disease that causes repeated episodes of rapid swelling, known as HAE attacks, in different parts of the body, including:
- hands and feet;
- face, eyelids, lips, or tongue;
- larynx and throat, which can make breathing difficult;
- genitals;
- stomach and intestine.
HAE attacks can be painful and debilitating. Attacks that affect the throat or larynx can be dangerous or even life-threatening.
Although HAE often occurs in families, some people may not have a family history. There are three types of HAE, depending on the type of genetic defect and its effect on a protein that circulates in the blood, called C1 esterase inhibitor (C1-INH). A person may have low levels of C1-INH in the body (HAE type I), dysfunctional C1-INH (HAE type II), or HAE with normal C1-INH function (HAE type III). The latter type is extremely rare. All three types produce the same clinical symptoms of localized swelling.
C1-INH regulates a process in the body that controls the production of an inflammatory substance called bradykinin. Overproduction of bradykinin causes swelling and inflammation in people with HAE.
The active substance of ANDEMBRY, garadacimab, blocks the activation of a protein known as factor XIIa (FXIIa), which is involved in stimulating the production of bradykinin. By blocking the activity of FXIIa, garadacimab reduces the level of bradykinin, thereby preventing HAE attacks. Some subcategories of HAE with normal C1-INH may not respond to treatment with garadacimab. Talk to your doctor if you have any concerns about your medication.
2. What you need to know before you use ANDEMBRY
Do not use ANDEMBRY
If you are allergic to garadacimab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
- Talk to your doctor, pharmacist, or nurse before you start using ANDEMBRY.
- If you have a severe allergic reaction to ANDEMBRY with symptoms such as hives, chest tightness, difficulty breathing, wheezing, low blood pressure, or anaphylaxis, tell your doctor, pharmacist, or nurse immediately.
- Treat an HAE attack with your usual rescue medication without taking additional doses of ANDEMBRY.
Keep a record
It is strongly recommended that each time you are given ANDEMBRY, you note the name and batch number of the medicine. This will help you keep a record of the batches used.
Lab tests
Tell your doctor if you are using ANDEMBRY before you have any lab tests to measure your blood clotting. This is because ANDEMBRY may interfere with some lab tests and give false results.
Children and adolescents
ANDEMBRY is not recommended for use in children under 12 years of age. This is because it has not been studied in this age group.
Other medicines and ANDEMBRY
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
ANDEMBRY is not known to affect other medicines or be affected by other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using ANDEMBRY. There is limited information on the safety of using ANDEMBRY during pregnancy and breastfeeding. As a precaution, it is recommended to avoid using ANDEMBRY during pregnancy. Your doctor will discuss the risks and benefits of taking this medicine with you.
Driving and using machines
This medicine has no or negligible influence on the ability to drive and use machines.
ANDEMBRY contains proline.
This medicine contains 19.3 mg of proline in each pre-filled pen, equivalent to 16.1 mg/ml. Proline may be harmful for patients with hyperprolinemia, a rare genetic disease in which proline accumulates in the body. If you (or your child) have hyperprolinemia, do not use this medicine unless your doctor recommends it.
ANDEMBRY contains polysorbate 80.
This medicine contains 0.24 mg of polysorbate 80 in each pre-filled pen, which is equivalent to 0.2 mg/ml. Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergies.
3. How to use ANDEMBRY
ANDEMBRY is provided in a single-use pre-filled pen. Your treatment will start under the supervision and management of a healthcare professional.
Follow the instructions for administration of the medicine contained in this leaflet or as directed by your doctor, pharmacist, or nurse. If you are in doubt or have any other questions about the use of this medicine, consult your doctor, pharmacist, or nurse again.
How much ANDEMBRY to use
The recommended dose of ANDEMBRY is an initial loading dose of 400 mg administered in two 200 mg injections on the first day of treatment, followed by a monthly dose of 200 mg.
How to inject ANDEMBRY
You can administer ANDEMBRY yourself or a caregiver can administer it to you. In both cases, you or your caregiver must read and follow the instructions in section 7, "Instructions for use", carefully.
- ANDEMBRY is administered subcutaneously (under the skin) in the abdomen, thigh, or upper arm.
- A doctor, pharmacist, or nurse must show you how to administer ANDEMBRY correctly before you use it for the first time. Do not self-administer or allow a caregiver to administer until you have received training on how to inject the medicine.
- Use each pre-filled pen only once.
- If the pen does not work as expected, tell your doctor, pharmacist, or nurse as soon as possible.
- It is recommended to rotate injection sites.
If you use more ANDEMBRY than you should
Tell your doctor, pharmacist, or nurse if you use too much ANDEMBRY.
If you forget to use ANDEMBRY
If you miss a dose of ANDEMBRY, administer your dose as soon as possible. If you are not sure when to inject ANDEMBRY after a missed dose, consult your doctor, pharmacist, or nurse.
If you stop using ANDEMBRY
It is important that you keep using ANDEMBRY as directed by your doctor, even if you feel better.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects.
Common(may affect up to 1 in 10 people):
- Injection site reactions including redness, bruising, itching, and hives
- Headache
- Abdominal pain
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of ANDEMBRY
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer carton and on the label after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (2°C - 8°C). Do not freeze. Keep the pre-filled pen in the outer packaging to protect it from light.
The pre-filled pen can be stored at room temperature (up to 25°C) for a single period of up to 2 months, but not beyond the expiry date.
Do not store ANDEMBRY in the refrigerator after it has been stored at room temperature.
Do not use this medicine if you notice signs of deterioration such as particles or color change of the solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
ANDEMBRY Composition
- The active ingredient is garadacimab. Each pre-filled pen contains 200 mg of garadacimab in 1.2 ml of solution.
- The other components are histidine, arginine monohydrochloride, proline, polysorbate 80, and water for injectable preparations; see section 2 "ANDEMBRY contains proline and polysorbate 80".
Appearance of ANDEMBRY and Container Contents
ANDEMBRY is presented as a yellowish-brown to yellow, slightly opalescent to transparent injectable solution in a pre-filled pen.
ANDEMBRY is presented in individual containers containing a 1.2 ml pre-filled pen and in multiple packs of 3 packs, each containing 1 pre-filled pen.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
CSL Behring GmbH
Emil-von-Behring-Strasse 76
D-35041 Marburg
Germany
You can request more information about this medicinal product from the local representative of the marketing authorization holder:
België/Belgique/Belgien CSL Behring NV Tél/Tel: +32 15 28 89 20 | Lietuva CentralPharma Communications UAB Tel: +370 5 243 0444 |
| Luxembourg/Luxemburg CSL Behring NV Tél/Tel: +32 15 28 89 20 |
Ceská republika CSL Behring s.r.o. Tel: +420 702 137 233 | Magyarország CSL Behring Kft. Tel: +36 1 213 4290 |
Danmark CSL Behring AB Tlf: +46 8 544 966 70 | Malta AM Mangion Ltd. Tel: +356 2397 6333 |
Deutschland CSL Behring GmbH Tel: +49 6190 75 84810 | Nederland CSL Behring BV Tel: +31 85 111 96 00 |
Eesti CentralPharma Communications OÜ Tel: +3726015540 | Norge CSL Behring AB Tlf: +46 8 544 966 70 |
Ελλáδα CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Österreich CSL Behring GmbH Tel: +43 1 80101 1040 |
España CSL Behring S. A. Tel: +34 933 67 1870 | Polska CSL Behring Sp. z o.o. Tel.: +48 22 213 22 65 |
Francia CSL Behring SA Tél: +33 1 53 58 54 00 | Portugal CSL Behring Lda Tel: +351 21 782 62 30 |
Hrvatska Marti Farm d.o.o. Tel: +385 1 5588297 | România Prisum Healthcare S.R.L. Tel: +40 21 322 01 71 |
Ireland CSL Behring GmbH Tel: +49 69 305 17254 | Slovenija EMMES BIOPHARMA GLOBAL s.r.o. - podružnica v Sloveniji Tel: +386 41 42 0002 |
Ísland CSL Behring AB Sími: +46 8 544 966 70 | Slovenská republika CSL Behring Slovakia s.r.o. Tel: +421 911 653 862 |
Italia CSL Behring S.p.A. Tel: +39 02 34964 200 | Suomi/Finland CSL Behring AB Puh/Tel: +46 8 544 966 70 |
Κúπρος CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Sverige CSL Behring AB Tel: +46 8 544 966 70 |
Latvija CentralPharma Communications SIA Tel: +371 6 7450497 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu There are also links to other websites on rare diseases and orphan medicines.
- Instructions for Use
ANDEMBRY Solution for Injection in
Pre-filled Pen
Subcutaneous Use
Important:
This pre-filled pen works differently from other subcutaneous injection devices. Read the instructions for use carefully before using it and every time you receive a new pre-filled pen, as there may be updated information. This information does not replace consultation with your healthcare professional about your disease or treatment. In adolescent patients, ANDEMBRY should be administered under adult supervision. Make sure you have received training from your healthcare professional before using this pre-filled pen for the first time. |
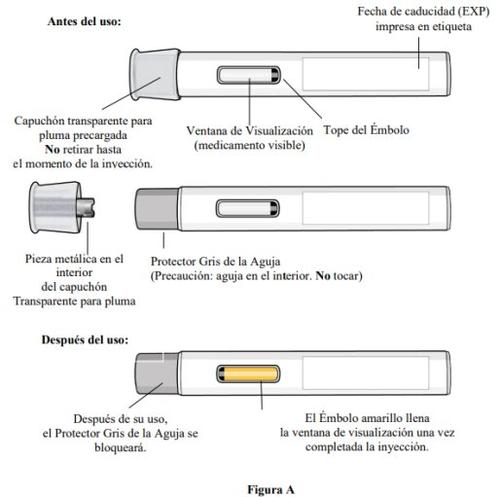
Parts of the Pen (see Figure A):
Continue with the following sections to prepare and administer the injection.
Read the following safety information:
- Keep the pre-filled pen in its original carton until use, to protect it from light.
- Do notremove the transparent cap from the pre-filled pen until the time of injection.
- Do notreplace the transparent cap of the pre-filled pen after removal, as this could initiate the injection and cause injury.
- The pre-filled pen contains 1 dose and is for single use. Do notreuse the same pre-filled pen.
- Do notuse the pre-filled pen if the expiration date has been exceeded.
- The pre-filled pen is for subcutaneous injection (under the skin) only.
- Do notuse the pre-filled pen if it appears damaged, has cracks, leaks medication, or has fallen. In these cases, discard the pre-filled pen as described in step 11. Disposal of the pre-filled penand use a new one.
- Do notadminister the medication through clothing.
- Do nottouch or attempt to remove the gray needle protector at any time.
- Keep ANDEMBRY out of sight and reach of children.
Contact your healthcare professional if you have any questions.
How should I store ANDEMBRY?
- Store ANDEMBRY pre-filled pen in the refrigerator, between 2 °C and 8 °C, in its original carton until use, to protect it from light.
- Do notfreeze. If the pre-filled pen has been frozen, do not useit even if it has been thawed.
- Remove the pre-filled pen from the refrigerator 30 minutes before use, to allow it to reach room temperature.
Alternative Storage (Room Temperature):
- In case of need, for example, when traveling, the pre-filled pen may be stored at room temperature (up to 25 °C) for a single period of up to 2 months, but not beyond the expiration date.
- If you decide to store the pre-filled pen at room temperature:
- In the space provided on the carton, write the date you first removed the pre-filled pen from the refrigerator to help you keep track of how long it has been stored at room temperature.
- Do notrefrigerate the pre-filled pen again after it has reached room temperature.
- Discard the pre-filled pen if it has been stored at room temperature for more than 2 months (see step 11. Disposal of the pre-filled pen).
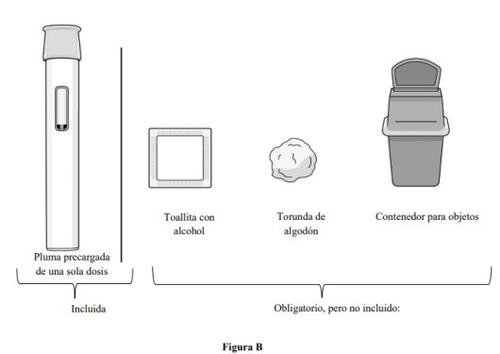
Supplies Needed for Injection with Pre-filled Pen (see Figure B):
Included in the carton:
- 1 single-dose pre-filled pen
Supplies needed, but not included in the carton:
- 1 alcohol swab
- 1 cotton ball or gauze
- 1 puncture-resistant container for disposal (see step 11. Disposal of the pre-filled pen).
Preparation for Administration
Do not remove the transparent cap from the pre-filled pen until just before administration.
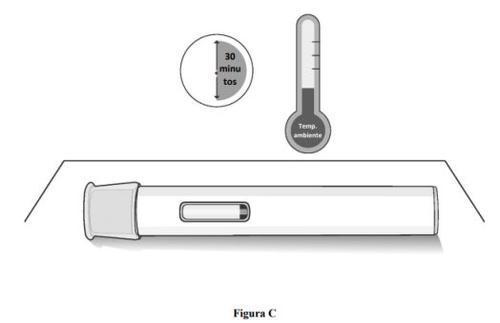
Step 1. Allow the pre-filled pen to reach room temperature.
- Remove the pre-filled pen from the carton and place it on a flat, clean surface.
- Wait 30 minutesfor the medication to reach room temperature if it has been stored in the refrigerator (see Figure C).
- Injecting cold medication may cause discomfort.
- Do notattempt to speed up the warming process in any way. For example, do notheat it in the microwave, in hot water, or expose it to direct sunlight.
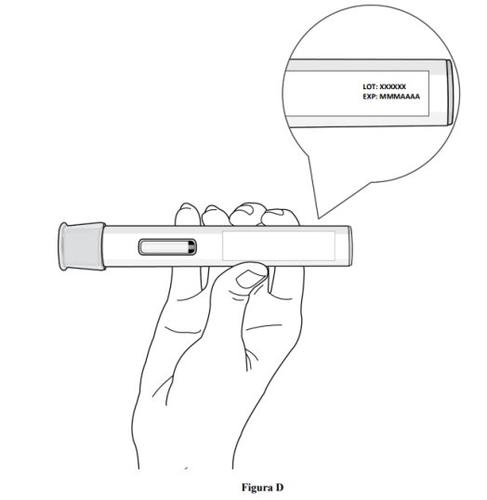
Step 2. Check the Expiration Date
- Check the expiration date on the pre-filled pen label (see Figure D).
- Do not usethe pre-filled pen if the expiration date has been exceeded.
- Do not usethe pre-filled pen if it has been stored at room temperature for more than 2 months.
- If the expiration date has been exceeded or if it has been stored at room temperature for more than 2 months, discard the pre-filled pen safely and use a new one (see step 11. Disposal of the pre-filled pen).
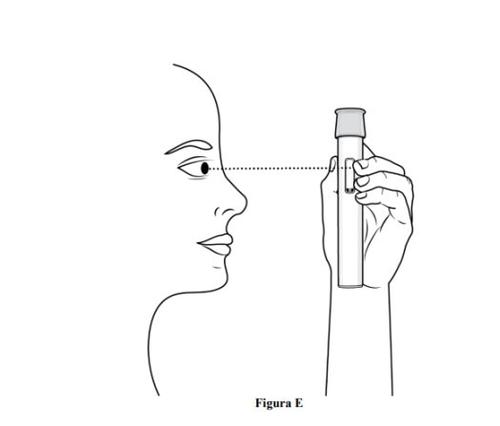
Step 3. Inspect the Pre-filled Pen and Medication
- Checkthat the pre-filled pen is not damaged.
- Inspectthe medicationthrough the viewing window of the pre-filled pen (see Figure E).
- It is normal to see air bubbles, do notattempt to remove them.
- The medication should be yellowish-brown to yellow and may appear slightly opalescent to transparent.
- Do not usethe pre-filled pen, discard it safely and use a new one (see step 11. Disposal of the pre-filled pen) if:
- The medication is discolored or contains particles.
- The pre-filled pen appears damaged or has cracks.
- The pre-filled pen is leaking.
- The pre-filled pen has fallen onto a hard surface, even if it does not appear damaged.
Choose and Prepare an Injection Site
Step 4. Wash Your Hands.
- Wash your hands with soap and water or use a disinfectant solution (see Figure F).
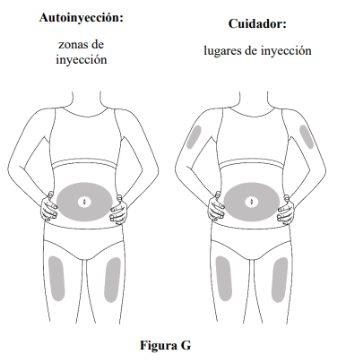
Step 5. Select the Injection Site
- Inject into the thigh or abdomen, but stay 2 cm away from the navel (see Figure G).
- If another person (caregiver) is administering the injection, they can also use the upper arm. Do notattempt to inject yourself in the upper arm.
- Change (i.e., rotate) the injection site with each injection. Do not injectinto the same area multiple times if the skin is damaged.
- Do notinject into the navel, moles, scars, or bruises, or into areas where the skin is sensitive, red, hard, or damaged.
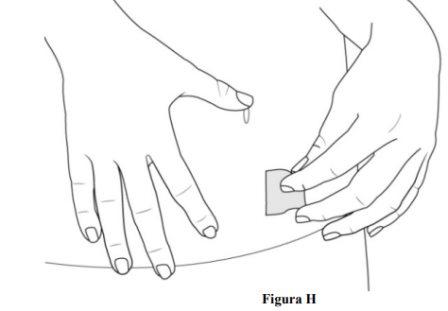
Step 6. Prepare the Injection Site
- Clean the injection site with an alcohol swab (see Figure H).
- Allow the skin to dry.
- Do nottouch this area again before administration.
- Do notfan or blow on the cleaned skin area.
Injecting the Medication with the Pre-filled Pen
Complete the injection without pauses. Read all the steps before starting. Do notremove the transparent cap until the time of injection. |
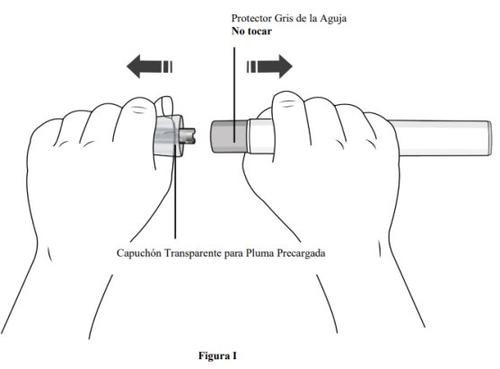
Step 7. Remove the Transparent Cap from the Pre-filled Pen and Discard
- Hold the pre-filled pen with one hand and removethe transparent cap from the pre-filled pen by pulling itwith the other hand.
- Do nottwist the transparent cap (see Figure I). If you cannot remove the transparent cap, ask for help from a doctor or contact your healthcare professional.
- The transparent cap has a metal part inside, this is normal.
- Do notreplace the transparent capafter removal, as this could initiate the injection and cause injury.
- Discard the needle cap in a puncture-resistant container.
Important:
|
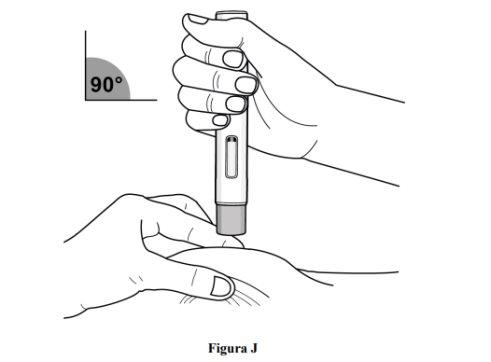
Step 8. Pinch the Skin and Place the Pre-filled Pen at the Injection Site
Immediately after removing the transparent cap from the pre-filled pen, complete the following steps without stopping:
- Gently pinch the clean skin around the injection site and hold the area firmly until the injection is complete (see Figure J).
- Place the pre-filled pen at a 90° angle to the injection site (see Figure J).
- Make sure you can see the viewing window.
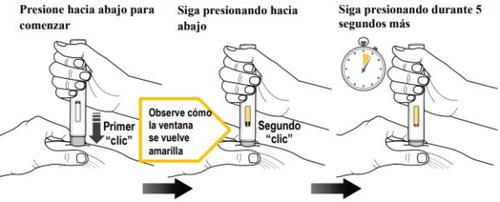
Step 9. Inject the Medication(see Figure K)
| You must read the entire contents of step 9 before administration. The injection may take up to 15 seconds. To ensure you receive a complete dose, you must hold the pre-filled pen firmly against the pinched skin until:
and
|
Press the gray needle protector firmly against the pinched skin to initiate administration and continue pressing until all the following steps are completed. | ||
| ||
Press down to startthe injection and wait for a first "click". | Keep pressingthe pre-filled pen downwardand observe the viewing window. | Keep pressingthe pre-filled pen downwardfor 5 seconds more to ensure you receive the complete dose. |
|
yellow and
| |
Continue pressingthe pre-filled pen downward. | Continue pressingthe pre-filled pen downward. |
Figure K
- Do notremove the pre-filled pen until the yellow plunger stops moving and completely fills the viewing window, and 5 seconds have passed after the second "click".
- Do notremove, tilt, or turn the pre-filled pen during the injection.
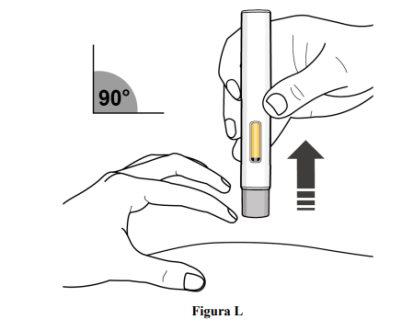
Step 10. Stop Pinching and Remove the Pre-filled Pen
- Stop pinching the skin and remove the pre-filled pen from the skin at a 90° angle (see Figure L).
- As the pre-filled pen is lifted off the skin, the gray needle protector will return to its original position (before use) and lock into place to cover the needle.
Important: If you think you have not received a complete dose, contact your healthcare provider immediately.
- If there is a little bleeding at the injection site, you can press a cotton ball or gauze over the injection site.
- Do notrub the injection site.
- injection site.
- If necessary, you can cover the administration point with a small adhesive bandage.
Disposal
Step 11. Disposal of the prefilled syringe
- Do notreuse the prefilled syringe.
- After administering the dose, place the prefilled syringe in a puncture-resistant container or a closed leak-resistant container (see Figure M).

- If you do not have a puncture-resistant container or a closed leak-resistant container, you can use a household container that is:
- Made of heavy-duty plastic.
- Can be closed with a tight-fitting, puncture-resistant lid, so that the sharp parts cannot come out.
- Stable during use.
- Leak-resistant.
- Properly labeled to warn of hazardous waste inside the container.
- When your puncture-resistant container is almost full, you will need to follow local guidelines for the correct way to dispose of it. Ask your pharmacist or healthcare professional for more information on how to dispose of your puncture-resistant container.
- Do notdispose of your used puncture-resistant container with household trash unless local guidelines permit it.
- Do notrecycle your used puncture-resistant container.
Step 12. Treatment follow-up
- If your doctor requires it, record the administration in a diary to help keep track of your medication.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ANDEMBRY 200 mg INJECTABLE SOLUTION IN PRE-FILLED PENDosage form: INJECTABLE, 1500 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 2000 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 3000 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription required
Online doctors for ANDEMBRY 200 mg INJECTABLE SOLUTION IN PRE-FILLED PEN
Discuss questions about ANDEMBRY 200 mg INJECTABLE SOLUTION IN PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions