ALKINDI 2 mg GRANULES IN CAPSULES TO OPEN

How to use ALKINDI 2 mg GRANULES IN CAPSULES TO OPEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: Information for the User
Alkindi 0.5mg granules in capsules to be opened
Alkindi 1mg granules in capsules to be opened
Alkindi 2mg granules in capsules to be opened
Alkindi 5mg granules in capsules to be opened
Hydrocortisone

Read the entire leaflet carefully before starting to administer this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medicine has been prescribed only for your child and should not be given to others. It could harm them, even if they have the same symptoms as the child for whom this medicine has been prescribed.
- If your child experiences any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Leaflet
- What is Alkindi and what is it used for
- What you need to know before starting to use Alkindi
- How to administer Alkindi
- Possible side effects
- Storage of Alkindi
- Package contents and additional information
1. What is Alkindi and what is it used for
Alkindi contains a medicine called hydrocortisone. Hydrocortisone belongs to a group of medicines called corticosteroids.
Hydrocortisone is a synthetic version of the hormone cortisol. Cortisol is naturally produced in the body by the adrenal glands. Alkindi is used when the body does not produce enough cortisol due to a non-functioning part of the adrenal gland (adrenal insufficiency, often due to a hereditary disorder called congenital adrenal hyperplasia).
2. What you need to know before starting to use Alkindi
Do not administer Alkindi:
- If your child is allergic to hydrocortisone or any of the other components of this medicine (listed in section 6).
- If your child has difficulty swallowing food or is a premature baby who is not yet fed by mouth.
Warnings and precautions
Consult your doctor or pharmacist before using Alkindi if your child:
- is ill or has an infection. The endocrinologist may temporarily increase the dose of Alkindi; consult the endocrinologist if your child becomes ill. If your child vomits or has an acute condition, they may need an injection of hydrocortisone. The endocrinologist will teach you how to administer it in case of an emergency.
- needs to be vaccinated. The administration of Alkindi does not prevent your child from being vaccinated. Inform your endocrinologist about your child's vaccination schedule.
- needs to undergo surgery. Inform the anesthesiologist that your child is taking Alkindi before the procedure.
- is fed through a nasogastric tube. The Alkindi granules are not suitable for administration through a nasogastric tube, as the granules may clog the tube.
Do not stop administering Alkindi without consulting your endocrinologist, as your child may become seriously ill quickly.
As Alkindi replaces the missing hormone, side effects are rare, but:
- An excess of Alkindi may affect your child's growth, so your endocrinologist will adjust the dose based on your child's size and closely monitor their growth. Talk to your endocrinologist if you are concerned about your child's growth rate (see section 4).
- An excess of Alkindi may affect your child's bones, so your endocrinologist will adjust the dose based on your child's size.
- Some adults taking hydrocortisone (similar to Alkindi) experience anxiety, depression, or confusion. It is not known if this can happen to children, but if your child shows abnormal behavior after starting Alkindi, discuss it with your endocrinologist (see section 4).
- Allergy to hydrocortisone has been observed in patients allergic to other medicines. Inform your endocrinologist immediately if your child experiences any reaction such as inflammation or difficulty breathing after receiving Alkindi (see section 4).
- Contact your endocrinologist if your child experiences blurred vision or other visual disturbances.
Sometimes, Alkindi granules may appear in your child's diaper or stool after taking Alkindi. This is because the core of the granules is not absorbed in the intestine once the medicine has been released. This does not mean that the medicine has not taken effect, so do not give your child another dose.
Other medicines and Alkindi
Tell your doctor or pharmacist if your child is taking, has recently taken, or might take any other medicines, including those that can be obtained without a prescription.
Some medicines affect the functioning of Alkindi, which may mean that your endocrinologist needs to adjust the dose of Alkindi your child receives.
Among the medicines that may require your endocrinologist to increase the dose of Alkindi your child receives are:
- Medicines used to treat epilepsy: phenytoin, carbamazepine, and oxcarbazepine.
- Medicines used to treat infections (antibiotics): rifampicin and rifabutin.
- Medicines called barbiturates, which can be used to treat seizures (such as phenobarbital and primidone).
- Medicines used to treat HIV and AIDS: efavirenz and nevirapine.
Among the medicines that may require your endocrinologist to reduce the dose of Alkindi your child receives are:
- Medicines used to treat fungal infections: itraconazole, posaconazole, and voriconazole.
- Medicines used to treat infections (antibiotics): erythromycin and clarithromycin.
- Medicine used to treat HIV and AIDS: ritonavir.
Taking Alkindi with food and drinks
Some foods and drinks affect the functioning of Alkindi, which may mean that your endocrinologist needs to reduce the dose of Alkindi your child receives. These include:
- Grapefruit juice.
- Licorice.
Pregnancy, breastfeeding, and fertility
Hydrocortisone can be used during pregnancy and breastfeeding if the body does not produce enough cortisol.
There are no data on the possible effects of Alkindi on fertility.
Driving and using machines
Alkindi does not affect your child's ability to perform tasks that require skill (such as riding a bike) or to use machines.
3. How to administer Alkindi
Follow the administration instructions for this medicine exactly as indicated by your endocrinologist, nurse, or pharmacist. If you are unsure, ask your endocrinologist, nurse, or pharmacist.
Your endocrinologist will decide the appropriate dose of Alkindi based on your child's weight or size (body surface area) and will adjust the dose of Alkindi as your child grows. During illnesses, before surgery, and in situations of intense stress, your endocrinologist may recommend increasing the dose of Alkindi and may also recommend that your child receive other forms of hydrocortisone instead of or in addition to Alkindi.
How to administer this medicine
The granules should be placed in the mouth and not chewed. The capsule shell should not be swallowed, but carefully opened as follows:
How to open Alkindi capsules and administer the granules |
|
|
|
|
|
|
| ||
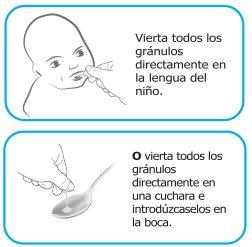
| Regardless of the method used, tap the capsule gently to ensure all the granules are released. If you administer the granules directly into the mouth, give your child something to drink (such as water, breast milk, or infant formula) immediately afterwards to ensure they swallow all the granules. If you sprinkle the granules over a spoonful of soft food, give it to your child immediately (within 5 minutes) and do not save it for future use DO NOTadd the granules to a liquid before administration, as this may result in a lower dose being administered and may also dissolve the flavor mask of the granules, making the bitter taste of hydrocortisone more noticeable. | |
|
|
If you administer more Alkindi than you should
If you have given your child more Alkindi than they should take, contact your endocrinologist or pharmacist as soon as possible for advice on what to do.
If you forget to administer Alkindi
If you forget to administer a dose to your child, give them the dose as soon as you remember and the next dose at the usual time, even if this means your child receives two doses at the same time.
If you stop treatment with Alkindi
Do not stop administering Alkindi to your child without consulting your endocrinologist. Stopping the medicine abruptly can make your child seriously ill quickly.
If your child becomes ill
Consult your endocrinologist or pharmacist if your child becomes ill, experiences intense stress, is injured, or is going to undergo surgery, as your endocrinologist may need to increase the dose of Alkindi in these circumstances (see section 2).
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
- If your child experiences any reaction such as inflammation or difficulty breathing after receiving Alkindi, seek medical attention immediately and inform your endocrinologist as soon as possible, as it could be an allergic reaction (see section 2).
No side effects have been observed with Alkindi, although the following side effects have been reported with other hydrocortisone medicines used to replace cortisol:
Frequency not known (cannot be estimated from the available data):
- Changes in behavior, such as:
- loss of contact with reality (psychosis) with sensations that are not real (hallucinations) and mental confusion (confusional syndrome).
- excessive excitement and activity (mania).
- intense feeling of happiness and excitement (euphoria).
If your child experiences a drastic change in behavior, consult your endocrinologist (see section 2).
- Stomach pain (gastritis) or nausea.
Contact your endocrinologist if your child reports any of these symptoms.
- Changes in potassium levels in the blood, which can cause an excess of alkalinity in the body's tissues or fluids (hypokalemic alkalosis).
Your endocrinologist will monitor your child's potassium levels to check for changes.
Long-term treatment with hydrocortisone may be associated with changes in bone development and a reduction in growth. Your endocrinologist will monitor your child's growth and bone health (see section 2).
Reporting side effects
If your child experiences any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Alkindi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the bottle and carton after EXP. The expiry date is the last day of the month indicated.
Do not store above 30°C. Store in the original bottle to protect from light.
Once the bottle is opened, use the capsules within 60 days.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package contents and additional information
Composition of Alkindi
- The active ingredient is hydrocortisone.
- Alkindi 0.5 mg granules in capsules to be opened: each capsule contains 0.5 mg of hydrocortisone
- Alkindi 1 mg granules in capsules to be opened: each capsule contains 1 mg of hydrocortisone
- Alkindi 2 mg granules in capsules to be opened: each capsule contains 2 mg of hydrocortisone
- Alkindi 5 mg granules in capsules to be opened: each capsule contains 5 mg of hydrocortisone
- The other ingredients are microcrystalline cellulose, hypromellose, magnesium stearate, and ethylcellulose.
- The capsule is made of hypromellose.
- The printing ink used on the capsules contains shellac, propylene glycol, and concentrated ammonia. The ink used on the 0.5 mg capsule also contains potassium hydroxide and red iron oxide (E172). The ink used on the 1 mg capsule also contains indigotine (E132). The ink used on the 2 mg capsule also contains indigotine (E132), yellow iron oxide (E172), and titanium dioxide (E171). The ink used on the 5 mg capsule also contains potassium hydroxide, titanium dioxide (E171), and red iron oxide (E172).
Appearance of Alkindi and package contents
White to off-white granules in a hard, transparent, and colorless capsule to be opened; the concentration is printed on the capsule.
- Alkindi 0.5 mg granules in capsules to be opened: the capsules are printed with the inscription "INF-0.5" in red ink.
- Alkindi 1 mg granules in capsules to be opened: the capsules are printed with the inscription "INF-1.0" in blue ink.
- Alkindi 2 mg granules in capsules to be opened: the capsules are printed with the inscription "INF-2.0" in green ink.
- Alkindi 5 mg granules in capsules to be opened: the capsules are printed with the inscription "INF-5.0" in gray ink.
Alkindi is available in high-density polyethylene bottles containing 50 capsules. Each carton contains 1 bottle.
Marketing authorization holder
Diurnal Europe B.V.
Van Heuven Goedhartlaan 935 A
1181LD Amstelveen
Netherlands
Tel.: +31 (0)20 6615 072
Manufacturer
Delpharm Lille SAS
Parc d'Activités Roubaix-Est
22 rue de Toufflers CS 50070
Lys Lez Lannoy, 59 452
France
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ALKINDI 2 mg GRANULES IN CAPSULES TO OPENDosage form: INJECTABLE, 373 mgActive substance: hydrocortisoneManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: INJECTABLE, 75 mgActive substance: hydrocortisoneManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: CAPSULE, 0.5 mgActive substance: hydrocortisoneManufacturer: Diurnal Europe B.V.Prescription required
Online doctors for ALKINDI 2 mg GRANULES IN CAPSULES TO OPEN
Discuss questions about ALKINDI 2 mg GRANULES IN CAPSULES TO OPEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions




 Hold the capsule so that the text is at the top and tap it gently to ensure the granules are at the bottom
Hold the capsule so that the text is at the top and tap it gently to ensure the granules are at the bottom Gently press the lower part of the capsule
Gently press the lower part of the capsule Twist and separate the top part of the capsule
Twist and separate the top part of the capsule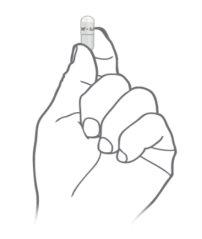
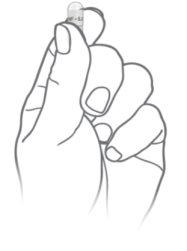
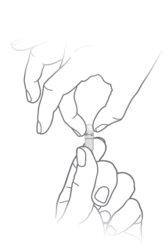
 Pour all the granules out of the capsule
Pour all the granules out of the capsule