
ALHEMO 60 mg/1.5 ml Injectable Solution in Pre-filled Pen

How to use ALHEMO 60 mg/1.5 ml Injectable Solution in Pre-filled Pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Alhemo 60mg/1.5ml solution for injection in a pre-filled pen
concizumab
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Alhemo and what is it used for
- What you need to know before you use Alhemo
- How to use Alhemo
- Possible side effects
- Storage of Alhemo
- Contents of the pack and other information
1. What is Alhemo and what is it used for
What is Alhemo
Alhemo contains the active substance concizumab, which belongs to a group of medicines called “monoclonal antibodies”. Concizumab is a protein that recognizes a target in the blood involved in the blood clotting process and binds to it.
What Alhemo is used for
Alhemo is used to prevent or reduce the frequency of bleeding episodes in adults and adolescents from 12 years of age with:
- Haemophilia A with inhibitors.
- Haemophilia B with inhibitors.
Haemophilia A is a congenital deficiency of factor VIII of blood coagulation and haemophilia B is a congenital deficiency of factor IX of blood coagulation.
How Alhemo works
Concizumab blocks a natural factor in the blood that prevents it from clotting. This factor is called tissue factor pathway inhibitor. This blockage makes blood clotting more efficient and, therefore, helps to prevent or reduce bleeding episodes when a clotting factor is lacking.
Alhemo works independently of factor VIII and factor IX and independently of inhibitors of these factors.
2. What you need to know before you use Alhemo
Do not use Alhemo
- if you are allergic to concizumab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Allergic reactions
There is a risk that you may experience an allergic reaction. Stop treatment and contact your doctor if you have symptoms of allergic reactions such as skin rash, redness, urticaria, and itching. You may also experience symptoms of severe allergic reactions such as:
- Itching over large areas of the skin
- Redness and/or swelling of the lips, tongue, face, or hands
- Difficulty swallowing
- Shortness of breath
- Wheezing
- Chest tightness
- Pale and cold skin
- Palpitations
- Dizziness due to low blood pressure.
Stop using Alhemo and seek immediate emergency helpif you have symptoms of severe allergic reactions.
Blood clots
Blood clots can form in any part of the body.
Stop using Alhemo and contact your doctor immediatelyif you have symptoms of blood clots such as:
- Swelling, warmth, pain, or redness of the skin: these may be symptoms of a blood clot in the legs or arms.
- Shortness of breath, severe chest pain: these may be symptoms of blood clots in the heart or lungs.
- Headache, feeling confused, problems with speech or movement, numbness in the face, pain or swelling in the eyes, or problems with vision: these may be symptoms of a blood clot in the brain or eyes.
- Sudden stomach or back pain: these may be symptoms of blood clots in the intestine or kidneys.
Children and adolescents
Alhemo is not recommended for children under 12 years of age with haemophilia A with inhibitors or haemophilia B with inhibitors.
Other medicines and Alhemo
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
Use a highly effective method of contraception during treatment with Alhemo and for 7 weeks after your last injection. Ask your doctor about the type of contraceptive you should use.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
Driving and using machines
Alhemo is unlikely to affect your ability to drive or use machines.
Alhemo contains sodium and polysorbates
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially “sodium-free”.
This medicine contains 0.25 mg of polysorbate 80 per ml. Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergies.
3. How to use Alhemo
Read the Instructions for Usecarefully on the other side of this leaflet before using the Alhemo pen. Ask your doctor if you need to use alternative injection techniques, e.g. it may be possible for thin patients and adolescents to inject into a skin fold to avoid injecting too deeply (into the muscle).
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, ask your doctor, pharmacist, or nurse.
Treatment of intercurrent bleeding episodes
Before starting treatment with Alhemo, ask your doctor about how to manage a possible bleeding episode.
The recommended dose for adults and adolescents is
- Initial dose on day 1: 1 mg per kg of body weight.
- From day 2 until the maintenance dose is established: 0.20 mg per kg of body weight once daily.
- Individual maintenance dose as decided by your doctor: 0.15; 0.20 or 0.25 mg per kg of body weight once daily.
Alhemo is injected under the skin of the abdomen or thigh and can be used at any time of day.
If you use more Alhemo than you should
Contact your doctor immediately.
If you forget to use Alhemo
Alhemo can be used at any time of day.
One or more missed doses of Alhemo may affect the efficacy of the medicine. It is important to administer Alhemo every day.
If you miss a dose of Alhemo:
If you miss a dose during the first 4 weeks of treatment, contact your doctor to agree on how to resume treatment.
If you miss a dose after the maintenance dose has been established:
- If you missed 1 daily dose, resume your daily dose.
- If you missed 2 to 6 daily doses, take your daily dose twice (as two separate injections each corresponding to a daily dose) and then continue with your daily dose the next day.
- If you missed 7 or more daily doses, contact your doctor immediately, as you will need to receive a new loading dose before continuing with your daily dose the next day.
If you have any doubts, contact your doctor.
If you stop treatment with Alhemo
You may no longer be protected against bleeding episodes. Do not stop treatment with Alhemo without consulting your doctor.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Alhemo and contact your doctor immediatelyif you have symptoms of severe allergic reactions or symptoms of blood clots.See “Warnings and precautions” in section 2.
Other side effects
Very common:may affect more than 1 in 10 people
- Injection site reactions such as redness, bleeding, itching, urticaria, swelling, pain, numbness.
Common:may affect up to 1 in 10 people
- Allergic reaction
Uncommon:may affect up to 1 in 100 people
- Blood clots
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Alhemo
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the pen and on the carton after EXP. The expiry date is the last day of the month shown.
Do not use this medicine if you notice any change in the color of the solution.
Before use
- Store unused Alhemo pens in the refrigerator at 2°C to 8°C.
- Store the new, unused pen with the cap on.
- When stored in the refrigerator, do not store the pen directly next to the cooling element.
- Do not use Alhemo if it has been frozen or stored at temperatures above 30°C.
After first use
- Store the Alhemo pen you are currently using without the needle attached for a maximum of 28 days (4 weeks), either:
- In the refrigerator at 2°C to 8°C.
Do not store the pen directly next to the cooling element. Do not use Alhemo if it has been frozen.
or
- At room temperature below 30°C.
Discard the pen if it has been stored at a temperature above 30°C. Do not store the pen in direct sunlight.
- Store the Alhemo pen in use with the cap on.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Alhemo
- The active ingredient is concizumab.
One milliliter of Alhemo 60 mg/1.5 ml contains 40 mg of concizumab.
- The other components are hydrochloride of L-arginine, L-histidine, sodium chloride, sucrose, polysorbate 80, phenol, hydrochloric acid/sodium hydroxide (for pH adjustment), and water for injectable preparations. Also, refer to section 2 "Alhemo contains sodium and polysorbates".
Appearance of the Product and Container Contents
Alhemo is a clear to slightly turbid and colorless to slightly yellowish injectable solution in a pre-filled pen. Translucent to white protein particles are acceptable.
Alhemo 60 mg/1.5 ml is available as a single-unit container containing 1 pre-filled pen or a multiple-unit container containing 5 (5 units of 1) pre-filled pens. Only certain pack sizes may be marketed.
Injection needles are not included.
Marketing Authorization Holder and Manufacturer
Novo Nordisk A/S
Novo Alle 1
DK-2880 Bagsvaerd
Denmark
Date of Last Revision of this Leaflet
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Instructions for Use
Alhemo 60mg/1.5ml Solution for Injection in Pre-filled Pen
Concizumab

What does this container contain?
- 1 Alhemo pen
- Leaflet
Needles are not included.
Read the instructions and make sure you have received training from your doctor or nurse before using the pen.
Follow your doctor's or nurse's instructions on how to use Alhemo and how often to inject Alhemo.
The pen is pre-filled with 60 mg of Alhemo for subcutaneous use (injection under the skin) only. The pen contains multiple doses of Alhemo.
The pen can deliver a maximum of 32 mg in one injection. The dose counter interval is 0.4 mg. If you need more than 32 mg, you must inject several times. There are other pens available that can deliver your daily dose in one injection. Ask your doctor or nurse.
Safety Information
The pen is for single-patient use and must not be shared. Sharing the pen or needles can cause infections and disease transmission.
Always use a new needle for each injection. Do not reuse needles, as this can cause needle blockage, infections, and incorrect dosing.
The needle is covered by two caps. You must remove both caps. If you forget to remove both caps, no solution will be injected.
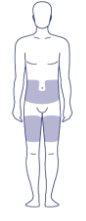
Where on my body should I inject the dose?
You can inject into the skin of:
- the stomach (abdomen) OR
- the thigh.
Inject at a 90° angle. The gray areas in the image on the right show the injection sites. For each injection, select a new injection site at least 5 centimeters away from the site of the last injection.
Check the Pen1
Check the Pen Label
Look at the name and color to make sure you have the correct medicine.
Inspect the Medicine
Remove the pen cap and check in the pen window that Alhemo is a clear to slightly turbid and colorless to slightly yellowish solution. Translucent to white protein particles are acceptable. If Alhemo looks discolored, do not use the pen.
Check the Expiration Date
Check the expiration date on the pen label to make sure it has not passed. Do not use the pen after the expiration date.
If the Pen is Cold
You can inject Alhemo directly from the refrigerator or let it reach room temperature before injecting. You can warm the pen with the palms of your hands. Do not use any other heat source.
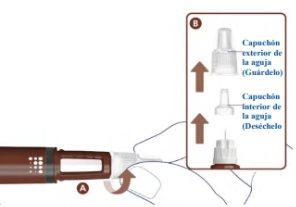
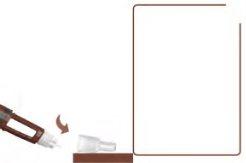
Attach a New Needle2
Take a new needle and remove the paper tab.
- Attach the needle straight to the pen. Screw it on until it is tight. See A.
- Remove the outer needle cap and set it aside. See B.
- Remove the inner needle cap and discard it. See B.
Never use a bent or damaged needle.
Use only needles recommended by your doctor or nurse. This pen is designed to be used with NovoFine Plus 32G x 4 mm or NovoFine 32G x 4 mm injection needles. If you use needles longer than 4 mm, ask your doctor or nurse how to perform the injection.
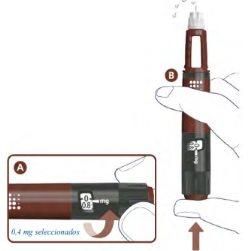
Check the Flow3
A drop of Alhemo may appear on the tip of the needle, but you must still check the flow of Alhemo before each injectionto avoid underdosing:
- Turn the dose selector one mark to select 0.4 mg. See A in the following figure. Hold the pen with the needle pointing up.
- Press the dose button. See B.
- Watch for liquid to appear at the needle tip. See B.
If no liquid appears, go to Troubleshooting if no liquid appears when checking the flow.
Select the Dose4
Turn the dose selector to select the prescribed dose.
Confirm that you have selected the correct dose.
You can adjust the dose by turning the dose selector in either direction.
If you need a dose higher than what you can dial, you must inject yourself more than once to get your full dose. There are other pens available that can deliver your daily dose in one injection. Ask your doctor or nurse. For more information, see step 6.
The pen contains 60 mg of Alhemo.
The pen can deliver a maximum of 32 mg in one injection.

Inject the Dose5
Read steps a. - e. before you start injecting.
This ensures that you get the full dose.
- Select the injection site. See Where on my body should I inject the dose?
- Insert the needle straight into the stomach (abdomen) or thigh at a 90° angle.
- Press and hold the dose button until the dose counter returns to <0>.
- With the needle still under the skin, slowly count to 6 after the dose counter has returned to<0>.
- Remove the needle from the skin.
The pen clicks during dose administration and may also click when the dose counter returns to <0>.
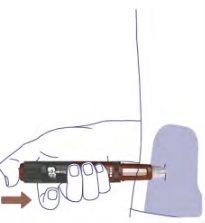
Remove the Needle6
Remove the needle from the pen after each injection by inserting the needle tip into the outer needle cap without touching the needle or the cap.
When the needle is covered, carefully press the outer needle cap down to the bottom. Unscrew the needle. Do not touch the back of the needle.
Dispose of the needle as instructed by your doctor, nurse, pharmacist, or local authorities.
Do you need a dose higher than what you can dial?
Your doctor should prescribe a pen that can deliver your daily dose in one injection. If you need a dose higher than what you can dial, you must inject yourself more than once to get your full dose.
Repeat steps 1 to 6 until you have received your full dose. When you have received your full dose, go to step 7.
- Always use a new needle for each injection.
- Check the flow of Alhemo before each injection.
- Accurately calculate the amount to be injected in each injection to receive the full dose.
After the Dose7
Put the cap back on the pen to protect Alhemo from light.
Now your pen is ready to be stored until you need it next.
After the first use, do not use the pen for more than 28 days.

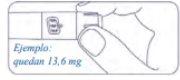
How much Alhemo is left in the pen?
The pen scale shows the approximate amount of Alhemo left in the pen.

If you want to see more precisely how much Alhemo is left in the pen, turn the dose selector until it stops. The dose marker will align with the number of milligrams left in the pen. The number shown on the dose counter is the number of milligrams left in the pen.
If the dose counter shows 32, there are 32 mg or more left in the pen. The following example shows 13.6 mg of Alhemo left in the pen.
Troubleshooting if no liquid appears when checking the flow (step 3)
- If no liquid appears, repeat step 3 up to six times until you see liquid.
- If still no liquid appears, prepare a new needle (step 2) and check again (step 3).
- If no liquid still appears after using a new needle, do not use the pen. Use a new pen.
Storage
Refer to section 5 "Storage of Alhemo" on the back of this leaflet.
Handle your Pen with Care
Treat the pen with care. Rough handling or misuse can cause inaccurate dosing. If this happens, you may not achieve the intended effect of this medicine.
Do not expose the pen to dust, dirt, or liquids.
Do not wash, wet, or lubricate the pen. If necessary, clean it with a damp cloth and a mild detergent.
Keep the pen out of sight and reach of others, especially children.
Disposal of Alhemo Pens, Needles, and Packaging Material
When the pen is empty, you must dispose of it according to local regulations.
The pen cannot be refilled.
To reduce the risk of needlestick injury, dispose of used needles immediately as instructed by your doctor, nurse, pharmacist, or local authorities.
[Text for the cover of the folded leaflet]
Leaflet and Instructions for Use
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ALHEMO 60 mg/1.5 ml Injectable Solution in Pre-filled PenDosage form: INJECTABLE, 100 mg/mlActive substance: concizumabManufacturer: Novo Nordisk A/SPrescription requiredDosage form: INJECTABLE, 100 mg/mlActive substance: concizumabManufacturer: Novo Nordisk A/SPrescription requiredDosage form: TABLET, 20 mgActive substance: avatrombopagManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription required
Online doctors for ALHEMO 60 mg/1.5 ml Injectable Solution in Pre-filled Pen
Discuss questions about ALHEMO 60 mg/1.5 ml Injectable Solution in Pre-filled Pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















