AJOVY 225 mg PRE-FILLED SYRINGE SOLUTION FOR INJECTION

How to use AJOVY 225 mg PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
AJOVY 225mg solution for injection in pre-filled syringe
fremanezumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is AJOVY and what is it used for
- What you need to know before you use AJOVY
- How to use AJOVY
- Possible side effects
- Storage of AJOVY
- Contents of the pack and other information
1. What is AJOVY and what is it used for
What is AJOVY
AJOVY is a medicine that contains the active substance fremanezumab, a monoclonal antibody, a type of protein that recognizes and binds to a specific target in the body.
How AJOVY works
A substance in the body called calcitonin gene-related peptide (CGRP) plays an important role in migraine. Fremanezumab binds to CGRP and prevents it from working. This reduction in CGRP activity reduces migraine attacks.
What AJOVY is used for
AJOVY is used to prevent migraine in adults with at least four days of migraine per month.
What are the benefits of using AJOVY
AJOVY reduces the frequency of migraine attacks and the number of days with headache. This medicine also reduces the disability associated with migraine and decreases the need to use medicines to treat migraine attacks.
2. What you need to know before you use AJOVY
Do not use AJOVY
Do not use this medicine if you are allergic to fremanezumab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Tell your doctor, pharmacist, or nurse immediately if you notice any signs of a severe allergic reaction such as difficulty breathing, swelling of the lips and tongue, or severe skin rash after injection of AJOVY. These reactions can occur within 24 hours of administration of AJOVY, although they can sometimes appear later.
Tell your doctor if you have or have had any cardiovascular disease (problems affecting the heart and blood vessels) before starting to use this medicine, as AJOVY has not been studied in patients with certain cardiovascular diseases.
Children and adolescents
AJOVY is not recommended for use in children and adolescents under 18 years of age, as it has not been studied in this age group.
Other medicines and AJOVY
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. It is preferable to avoid using AJOVY during pregnancy, as the effects of this medicine on pregnant women are unknown.
If you are breastfeeding or plan to breastfeed, consult your doctor or pharmacist before starting to use this medicine. You and your doctor will decide whether to use AJOVY during breastfeeding.
Driving and using machines
This medicine is not expected to have any effect on your ability to drive and use machines.
AJOVY contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially "sodium-free".
3. How to use AJOVY
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
Read carefully the "Instructions for use" of the pre-filled syringe before starting to use AJOVY.
How much and when to inject
Your doctor will discuss and decide with you the most suitable dosage regimen. There are two alternative dosage regimens recommended:
- one injection (225 mg) once a month (monthly dosage regimen) or
- three injections (675 mg) every three months (quarterly dosage regimen)
If your dose is 675 mg, administer the three injections one after the other, in a different place each time.
AJOVY is administered by injection under the skin (subcutaneous injection). Your doctor or nurse will explain to you or your caregiver how to administer the injection. Do not inject AJOVY until your doctor or nurse has taught you or your caregiver how to do it.
Use some reminder method, such as notes in a calendar or diary, to help you remember the next dose and not miss a dose or inject a dose too soon after the last one.
If you use more AJOVY than you should
If you have used more AJOVY than you should, consult your doctor.
If you forget to use AJOVY
If you have missed a dose of AJOVY, inject the missed dose as soon as possible. Do not administer a double dose to make up for missed doses. If you are unsure when you should inject AJOVY, consult your doctor, pharmacist, or nurse.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur:
Very common (may affect more than 1 in 10 people)
Pain, hardening, or redness at the injection site
Common (may affect up to 1 in 10 people)
Itching at the injection site
Uncommon (may affect up to 1 in 100 people)
Skin rash at the injection site
Allergic reactions such as exanthema, swelling, or urticaria
Rare (may affect up to 1 in 1,000 people)
Severe allergic reactions (signs may be difficulty breathing, swelling of the lips and tongue, or severe skin rash) (see section 2 "Warnings and precautions").
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of AJOVY
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the syringe and on the outer packaging after "EXP". The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the pre-filled syringe in the outer packaging to protect the medicine from light.
This medicine can be taken out of the refrigerator and stored at a temperature not above 30°C for a maximum of 7 days. The medicine should be discarded if it has been out of the refrigerator for more than 7 days. Once stored at room temperature, do not put it back in the refrigerator.
Do not use this medicine if you notice that the outer packaging has been tampered with, the syringe is damaged, or the medicine is cloudy, has changed color, or contains particles.
The syringe is for single use.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
AJOVY Composition
- The active ingredient is fremanezumab.
Each prefilled syringe contains 225 mg of fremanezumab.
- The other components (excipients) are L-histidine, L-histidine hydrochloride monohydrate, sucrose, disodium edetate dihydrate, polysorbate 80, and water for injectable preparations.
Product Appearance and Container Contents
AJOVY is an injectable solution in a prefilled syringe with a fixed needle in a blister. AJOVY is a clear, colorless to slightly yellow solution. Each prefilled syringe contains 1.5 ml of solution.
AJOVY is available in packs containing 1 or 3 prefilled syringes. Not all pack sizes may be available in your country.
Marketing Authorization Holder
TEVA GmbH
Graf-Arco-Str. 3
89079 Ulm
Germany
Manufacturer
Merckle GmbH
Graf-Arco-Str. 3
89079 Ulm
Germany
Teva Pharmaceuticals Europe B.V.
Swensweg 5
2031 GA Haarlem
Netherlands
You can request more information about this medicinal product from the local representative of the marketing authorization holder.
België/Belgique/Belgien Teva Pharma Belgium N.V./S.A./AG Tél/Tel: +32 38207373 | Lietuva UAB Teva Baltics Tel: +370 52660203 |
България Тева Фарма ЕООД Тел: +359 24899585 | Luxembourg/Luxemburg Teva Pharma Belgium N.V./S.A./AG Belgique/Belgien Tél/Tel: +32 38207373 |
Ceská republika Teva Pharmaceuticals CR, s.r.o. Tel: +420 251007111 | Magyarország Teva Gyógyszergyár Zrt. Tel: +36 12886400 |
Danmark Teva Denmark A/S Tlf: +45 44985511 | Malta Teva Pharmaceuticals Ireland Ireland Tel: +44 2075407117 |
Deutschland TEVA GmbH Tel: +49 73140208 | Nederland Teva Nederland B.V. Tel: +31 8000228400 |
Eesti UAB Teva Baltics Eesti filiaal Tel: +372 6610801 | Norge Teva Norway AS Tlf: +47 66775590 |
Ελλάδα ΤΕΒΑ ΕΛΛΑΣ Α.Ε. Τηλ: +30 2118805000 | Österreich ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1970070 |
España Teva Pharma, S.L.U. Tel: +34 913873280 | Polska Teva Pharmaceuticals Polska Sp. z o.o. Tel: +48 223459300 |
France Teva Santé Tél: +33 155917800 | Portugal Teva Pharma - Produtos Farmacêuticos, Lda. Tel: +351 214767550 |
Hrvatska Pliva Hrvatska d.o.o. Tel: +385 13720000 | România Teva Pharmaceuticals S.R.L. Tel: +40 212306524 |
Ireland Teva Pharmaceuticals Ireland Tel: +44 2075407117 | Slovenija Pliva Ljubljana d.o.o. Tel: +386 15890390 |
Ísland Teva Pharma Iceland ehf. Sími: +354 5503300 | Slovenská republika TEVA Pharmaceuticals Slovakia s.r.o. Tel: +421 257267911 |
Italia Teva Italia S.r.l. Tel: +39 028917981 | Suomi/Finland Teva Finland Oy Puh/Tel: +358 201805900 |
Κύπρος ΤΕΒΑ ΕΛΛΑΣ Α.Ε. Ελλάδα Τηλ: +30 2118805000 | Sverige Teva Sweden AB Tel: +46 42121100 |
Latvija UAB Teva Baltics filiale Latvija Tel: +371 67323666 | United Kingdom (Northern Ireland) Teva Pharmaceuticals Ireland Ireland Tel: +44 2075407117 |
Date of Last Revision of this Leaflet:{Month YYYY}.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
------------------------------------------------------------------------------------------------------------------------
Instructions for Use
AJOVY 225 mg Solution for Injection in Prefilled Syringe
fremanezumab
Before Using the AJOVY Prefilled Syringe, Read and Follow the Step-by-Step Instructions Carefully.
Important Information:
- Read the AJOVY leaflet carefully to obtain more information about the medicinal product.
- Do notpull the plunger at any time, as the prefilled syringe may break.
- Do notshake the prefilled syringe.
- Return the box to the refrigerator immediatelyif you have any unused prefilled syringes in the box.
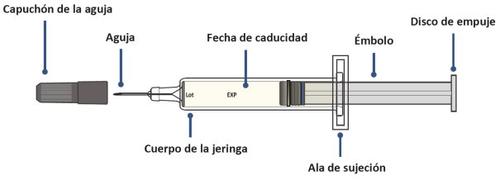
AJOVY Prefilled Syringe Components
Step 1: Preparation for Injection
- Gather the Following Materials for Your Injection:
- 1 or 3 AJOVY prefilled syringes to administer 1 or 3 injections depending on your dose
- 1 alcohol swab per injection
- 1 cotton ball or swab per injection
- 1 puncture-resistant container for disposal of sharp objects
- Place the Gathered Materials on a Clean, Flat Surface.
- Wait 30 Minutes to Allow AJOVY to Reach Room Temperature to Reduce Discomfort During Injection.
- Do notexpose the prefilled syringe to direct sunlight.
- Do notheat the prefilled syringe in a microwave or with any other heat source.
- Do notremove the needle cap.
- Wash Your Handswith water and soap and dry them well with a clean towel.
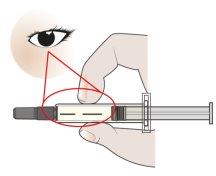
- Inspect the AJOVY Prefilled Syringe.
- Check the syringe label. Make sure the name AJOVY appears on the label.
- Check that the medicine inside the syringe is clear and colorless to slightly yellow.
- You may see small air bubbles in the prefilled syringe. This is normal.
- Do notuse the prefilled syringe if you notice any of the following:
- The syringe appears damaged.
- The expiry date has passed or the prefilled syringe has been out of the refrigerator for more than 7 days.
- The medicine is cloudy, has changed color, or contains particles.
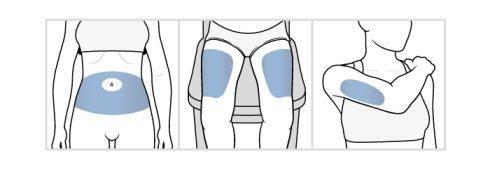
- Choose the Injection Site.
- Chooseone of the following injection sites:
- the abdomen(stomach area), avoiding a 5 cm margin around the navel
- the front of the thighs, about 5 cm above the knee and 5 cm below the groin
- the back of the arms, in the fleshy areas of the back of the arm
- If more than one injection is required, they can be administered in the same area or in different areas (abdomen, thigh, arm), but avoid administering them exactly in the same spot.
- Clean the Injection Site.
- Clean the chosen injection site using a new alcohol swab.
- Wait 10 seconds to allow the skin to dry before administering the injection.
- Do notinject AJOVY into an area that is painful, red, hot, bruised, hardened, or scarred.
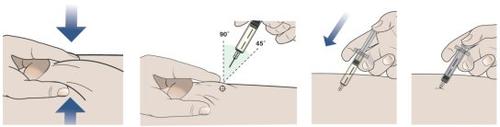
Step 2: How to Inject
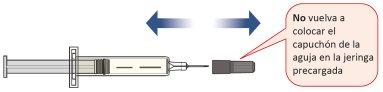
- Only When You Are Ready to Inject, Pull the Needle Cap Straight Off and Dispose of It.
- Do notreplace the needle cap on the prefilled syringe to avoid injury or infection.
- Do nottouch the needle.
- Inject Following the Four Steps Indicated Below.
|
|
|
|
|
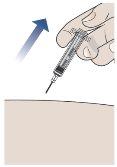
- Remove the Needle from the Skin.
- Once all the medicine has been injected, pull the needle straight out.
- Do notreplace the needle cap at any time to avoid injury or infection.
- Apply Pressure to the Injection Site.
- Use a clean, dry cotton ball or swab to apply gentle pressure to the injection site for a few seconds.
- Do notrub the injection site or reuse the prefilled syringe.
Step 3: Disposal of the Prefilled Syringe
- Discard the Prefilled Syringe Immediately.
- Throw away used prefilled syringes (with the needle still attached) in a puncture-resistant container right after use.
- Do notthrow away loose needles, syringes, or prefilled syringes in the trash.
- Do notrecycle the used puncture-resistant container.
- Ask Your Doctor, Pharmacist, or Nurse How to Dispose of the Container.
If Your Dose is 675 mg, Repeat Steps 1 to 3 with the Second and Third Prefilled Syringes to Inject the Full Dose.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AJOVY 225 mg PRE-FILLED SYRINGE SOLUTION FOR INJECTIONDosage form: INJECTABLE, 225 mgActive substance: fremanezumabManufacturer: Teva GmbhPrescription requiredDosage form: INJECTABLE, 140 mgActive substance: erenumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 70 mgActive substance: erenumabManufacturer: Novartis Europharm LimitedPrescription required
Online doctors for AJOVY 225 mg PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Discuss questions about AJOVY 225 mg PRE-FILLED SYRINGE SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions