
ACTIQ 1600 micrograms, ORAL LOZENGES WITH INTEGRATED ORAL APPLICATOR

How to use ACTIQ 1600 micrograms, ORAL LOZENGES WITH INTEGRATED ORAL APPLICATOR
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Actiq 200 micrograms lozenges with integrated oral applicator
Actiq 400 micrograms lozenges with integrated oral applicator
Actiq 600 micrograms lozenges with integrated oral applicator
Actiq 800 micrograms lozenges with integrated oral applicator
Actiq 1200 micrograms lozenges with integrated oral applicator
Actiq 1600 micrograms lozenges with integrated oral applicator
fentanyl
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is Actiq and what is it used for
- What you need to know before you use Actiq
- How to use Actiq
- Possible side effects
- Storing Actiq
- Contents of the pack and further information
1. What is Actiq and what is it used for
Actiq contains the active substance fentanyl, a potent analgesic belonging to the group of opioids. Actiq is presented as lozenges with an integrated oral applicator.
- Actiq is indicated for the treatment of breakthrough pain in adult patients and adolescents of 16 years and older with cancer, who are already receiving opioid therapy for their persistent (chronic) cancer pain. Breakthrough pain is a sudden increase in pain that occurs on top of a background of persistent pain, and that is not relieved by the opioid medication they are already taking.
2. What you need to know before you use Actiq
DO NOT use Actiq:
- if you are not already using a prescribed opioid medicine every day for your persistent cancer pain (e.g. codeine, fentanyl, hydromorphone, morphine, oxycodone, meperidine), for at least one week. If you have not been using these medicines, do not useActiq as its use can increase the risk that your breathing becomes slower and/or shallower and even stops.
- if you are allergic to fentanyl or any of the other ingredients of this medicine (listed in section 6).
- if you are currently taking monoamine oxidase inhibitors (MAOIs) (for the treatment of severe depression) or have taken them in the last 2 weeks (see section 2 “Consult your doctor or pharmacist BEFOREyou start using Actiq if”).
- if you are taking a medicine that contains sodium oxybate.
- if you have severe respiratory problems or severe obstructive pulmonary disease.
- if you have short-term pain (e.g. pain from injuries, surgery, headache or migraines) other than breakthrough cancer pain.
DO NOT use Actiq if you are in any of the above situations. If you are not sure, consult your doctor or pharmacist BEFOREusing Actiq.
Warnings and precautions
During treatment with Actiq, continue to use the opioid pain medicine you are taking for your persistent cancer pain.
Keep this medicine out of the sight and reach of children and in a safe place to prevent abuse or theft (see section 5 Storing Actiqfor more information).
Consult your doctor or pharmacist BEFOREyou start using Actiq if:
- The other opioid medicine you are taking for your persistent cancer pain has not yet been stabilized.
- You have any disease that affects your breathing (such as asthma, wheezing, or difficulty breathing).
- You have had a head injury or have had loss of consciousness.
- You have heart problems, especially slow heart rate.
- You have liver or kidney problems, as these affect how your body gets rid of the medicine.
- You have low blood pressure due to low blood volume in the circulatory system.
- You are diabetic.
- You are over 65 years old, as it may be necessary to reduce the dose and any increase in dose should be carefully supervised by your doctor.
- You are using benzodiazepines (see section 2 “Other medicines and Actiq”). The use of benzodiazepines can increase the risk of serious side effects, including death.
- You are using antidepressants or antipsychotics (selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs); see section 2 “DO NOT use Actiq” or “Other medicines and Actiq”. The use of these medicines with Actiq can cause a potentially life-threatening serotonin syndrome(see section 2 “Other medicines and Actiq”).
- You have ever abused or been dependent on opioids or any other medicine, alcohol, or illegal drugs.
- You have ever developed adrenal insufficiency, a condition in which the adrenal glands do not produce enough hormones, or a lack of sex hormones (androgen deficiency) with the use of opioids (see section 4, “Serious side effects”).
- You drink alcohol; see the section on Using Actiq with food, drinks, and alcohol.
Consult your doctor DURINGtreatment with Actiq if:
- You feel more sensitive to pain (hyperalgesia) that does not respond to a higher dose of the medicine as prescribed by your doctor.
- You have signs of dental caries. Actiq contains approximately 2 grams of sugar; frequent use exposes you to an increased risk of dental caries that can be serious. It is important to continue to take good care of your mouth and teeth during treatment with Actiq. Visit your dentist regularly during treatment.
- You experience a combination of the following symptoms: nausea, vomiting, loss of appetite, fatigue, weakness, dizziness, and low blood pressure. Together, these symptoms can be a sign of a potentially life-threatening condition called adrenal insufficiency, in which the adrenal glands do not produce enough hormones.
- Respiratory disorders related to sleep: Actiq can cause sleep-related respiratory disorders such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen level in the blood). Symptoms may include pauses in breathing during sleep, nighttime awakenings due to difficulty breathing, difficulty staying asleep, or excessive daytime sleepiness. If you or someone else observes these symptoms, contact your doctor. Your doctor may consider reducing the dose.
Long-term use and tolerance
This medicine contains fentanyl, an opioid. Repeated use of opioid analgesics can make the medicine less effective (the body gets used to it, which is known as pharmacological tolerance). It is also possible that you may become more sensitive to pain when using Actiq. This is known as hyperalgesia. Increasing the dose of Actiq may continue to reduce pain for a while, but it can also be harmful. If you notice that the medicine is becoming less effective, consult your doctor. Your doctor will decide whether it is best for you to increase the dose or gradually reduce the use of Actiq.
Dependence and addiction
This medicine contains fentanyl, which is an opioid. It can cause dependence and/or addiction.
Repeated use of Actiq can also lead to dependence, abuse, and addiction, which can result in a potentially life-threatening overdose. The risk of these side effects may be greater with higher doses and longer use. Dependence or addiction can cause a feeling of lack of control over the amount of medicine you need to use or how often you need to use it. You may feel the need to continue using the medicine even if it does not help relieve pain.
The risk of dependence or addiction varies from person to person. The risk of becoming dependent on or addicted to Actiq may be greater if:
- you or a family member have abused alcohol or experienced dependence on it, prescription medicines, or illegal drugs (“addiction”).
- you smoke.
- you have ever had mood problems (depression, anxiety, or personality disorder) or have received treatment from a psychiatrist for other mental health problems.
If you notice any of the following symptoms while using Actiq, it could be a sign of dependence or addiction.
- you need to use the medicine for longer than prescribed by your doctor.
- you need to use a higher dose than recommended.
- you are using the medicine for reasons other than those prescribed, for example, “to feel calm” or “to help you sleep”.
- you have made repeated unsuccessful attempts to stop using the medicine or control its use.
- you feel unwell when you stop using the medicine (e.g. nausea, vomiting, diarrhea, anxiety, shivers, tremors, and sweating), and you feel better once you take it again (“withdrawal symptoms”).
If you notice any of these signs, consult your doctor to determine the best treatment for you, when it is appropriate to stop the medicine, and how to do it safely.
Seek medical advice URGENTLYif:
- You notice symptoms such as difficulty breathing or dizziness, swelling of the tongue, lips, or throat while using Actiq. These can be the first symptoms of a severe allergic reaction (anaphylaxis, hypersensitivity; see section 4 “Serious side effects”).
Children and adolescents
Actiq is not recommended for children and adolescents under 16 years of age.
Use in athletes
This medicine contains fentanyl, which can produce a positive result in doping tests.
Other medicines and Actiq
Do not use this medicine and inform your doctor or pharmacist:
- If you are taking other fentanyl-based treatments that you have been prescribed previously for breakthrough pain. If you still have these fentanyl products at home, contact your pharmacist, who will tell you how to dispose of them.
- If you are taking monoamine oxidase inhibitors (MAOIs) (medicines for severe depression) or have taken them in the last two weeks (see section 2 “DO NOT use Actiq” and “Consult your doctor or pharmacist BEFOREyou start using Actiq if”).
Tell your doctor or pharmacist before using Actiq if you are taking or have recently taken or might need to take any other medicines. This includes medicines obtained without a prescription and herbal remedies. In particular, tell your doctor or pharmacist if you are using any of the following medicines:
- The concomitant use of Actiq and sedative medicines, such as benzodiazepines or related drugs, increases the risk of drowsiness, breathing difficulties (respiratory depression), and coma, and can put your life at risk. Therefore, concomitant use should only be considered when other treatment options are not possible.
However, if your doctor prescribes Actiq with sedative medicines, the dose and duration of concomitant treatment should be limited by your doctor.
- Tell your doctor about all sedative medicines you are taking (such as sleeping pills, medicines to treat anxiety, some medicines to treat allergic reactions (antihistamines), or tranquilizers) and strictly follow the recommended dose prescribed by your doctor. It may be helpful to inform friends or family members to alert them to the signs and symptoms described above. Contact your doctor if you experience these symptoms.
- Certain muscle relaxants, such as baclofen or diazepam (see also the section “Warnings and precautions”).
- Any medicine that may affect how your body gets rid of Actiq, such as ritonavir or other medicines that help control HIV infection (AIDS) or other medicines called “CYP3A4 inhibitors” such as ketoconazole, itraconazole, or fluconazole (used for fungal infections) and troleandomycin, clarithromycin, or erythromycin (medicines for bacterial infections) and “CYP3A4 inducers” such as rifampicin or rifabutin (medicines for bacterial infections), carbamazepine, phenobarbital, or phenytoin (medicines used to treat seizures/attacks)
- Certain potent pain relievers, called partial agonist/antagonists, such as buprenorphine, nalbuphine, and pentazocine (medicines for pain relief). While using these medicines, you may experience symptoms of a withdrawal syndrome (nausea, vomiting, diarrhea, anxiety, shivers, tremors, and sweating).
- Certain pain relievers for neuropathic pain (gabapentin and pregabalin).
- Serotoninergic medicines used to treat depression (antidepressants: such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) or antipsychotics. The use of these medicines with Actiq can lead to a potentially life-threatening serotonin syndrome(see section 2 “Consult your doctor or pharmacist BEFOREyou start using Actiq if” and “DO NOT use Actiq”). The symptoms of serotonin syndrome can include changes in mental status (e.g. agitation, hallucinations, coma) and other effects such as body temperature above 38°C, increased heart rate, unstable blood pressure, and exaggerated reflexes, muscle stiffness, lack of coordination, and/or gastrointestinal symptoms (e.g. nausea, vomiting, diarrhea). Your doctor will tell you if Actiq is suitable for you.
If you need to undergo surgery that requires general anesthesia, consult your doctor or nurse.
Using Actiq with food, drinks, and alcohol
- Actiq can be used before or after meals. However, do not use it during a meal.
- You can drink a little water before using Actiq to moisten your mouth. However, do not drink or eat anything while using Actiq.
- Do not drink grapefruit juice while using Actiq, as it may affect how your body gets rid of Actiq.
- Do not drink alcoholic beverages while being treated with Actiq. It can increase the risk of serious side effects, including death.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
Actiq should not be used during pregnancy unless you have discussed it with your doctor.
If Actiq is used for long periods during pregnancy, there is a risk that the newborn may suffer from withdrawal syndrome, which can be life-threatening if not recognized and treated by a doctor (see section 4 “Other side effects, frequency not known”).
Do not use Actiq during labor because it can cause breathing difficulties in the newborn.
Breastfeeding
Fentanyl can pass into breast milk and cause side effects in the breastfed child. Do not use Actiq if you are breastfeeding. You should not start breastfeeding until at least 5 days after the last dose of Actiq.
Driving and using machines
This medicine may affect your ability to drive or use certain tools or machines. Consult your doctor about the safety for you of driving or using certain tools or machines in the hours following the use of Actiq.
Do not drive or use machines if: you feel drowsy or dizzy; you have blurred or double vision; you have difficulty concentrating. It is important that you know how Actiq affects you before driving or using machines.
Actiq contains glucose
This medicine contains approximately 1.89 grams of glucose per dose. This should be taken into account in patients with diabetes mellitus. Patients with glucose or galactose absorption problems should not take this medicine.
It can cause tooth decay.
Actiq contains sucrose
This medicine contains sucrose. Patients with hereditary fructose intolerance (HFI), glucose or galactose absorption problems, or sucrase-isomaltase insufficiency should not take this medicine.
It can cause tooth decay.
Actiq contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per lozenge; it is essentially “sodium-free”.
3. How to use Actiq
Follow your doctor's instructions for taking this medication exactly. If you are in doubt, consult your doctor or pharmacist again.
Before starting treatment and regularly during treatment, your doctor will also explain what you can expect from using Actiq, when and for how long you should use it, when you should contact your doctor, and when you should stop using it (see also section 2).
When you start using Actiq for the first time, your doctor will work with you to find the dose of Actiq that relieves your breakthrough pain. It is very important that you use Actiq exactly as your doctor has instructed.
- Do not change the dose of Actiq or other painkillers on your own. Any change in dosage must be prescribed and monitored by your doctor.
- If you have doubts about the correct dose or questions about using Actiq, consult your doctor.
How the medicine enters the body
When you put Actiq in your mouth:
- The lozenge dissolves, and the active substance is released. This process takes place in about 15 minutes.
- The active substance is absorbed through the oral mucosa into the bloodstream.
The fact that you use the medicine in this way allows it to be absorbed quickly, which means rapid relief from breakthrough pain.
Determining the correct dose
You should start to feel relief quickly while using Actiq. However, until you and your doctor determine the dose that effectively controls breakthrough pain, you may not feel sufficient pain relief 30 minutes after starting to use a unit of Actiq (15 minutes after finishing the Actiq lozenge). If this happens, your doctor may allow you to use a second Actiq lozenge of the same dose to treat the same episode of breakthrough pain.
Do not use a second unit unless your doctor tells you to. Never use more than two units to treat a single episode of breakthrough pain.
During dose determination, you may need to have units of Actiq of different concentrations at home. However, keep only the concentrations of Actiq that you need at home. This helps prevent possible confusion and overdose. Consult your pharmacist on how to dispose of unused units of Actiq.
How many units should be used
Once you have determined the correct dose with your doctor, use 1 unit for an episode of breakthrough pain. Consult your doctor if your correct dose of Actiq does not relieve breakthrough pain over several consecutive episodes of breakthrough pain. Your doctor will decide if it is necessary to change the dose.
You must inform your doctor immediately if you use Actiq more than four times a day, as you may need a change in your treatment regimen. Your doctor may change the treatment for persistent pain; when the persistent pain is controlled, your doctor may need to change the dose of Actiq. If your doctor suspects increased sensitivity to pain related to Actiq (hyperalgesia), a reduction in your dose of Actiq may be considered (see section 2 "Warnings and precautions"). To get the best results, inform your doctor about the pain you are experiencing and how Actiq is working, so the dose can be changed if necessary.
Using the medicine
Opening the packaging -Each unit of Actiq is sealed in its own blister packaging.
- Open the packaging when you are ready to use it. Do not open it beforehand.
- Hold the blister packaging with the printed side facing away from you.
- Hold the short end of the blister packaging.
- Place the scissors near the end of the Actiq unit and cut off the long end completely (see the illustration).
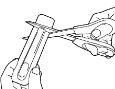
- Separate the printed back of the blister packaging and pull it out completely.
- Remove the Actiq unit from the blister packaging and immediately place the Actiq lozenge in your mouth.
Using the Actiq unit
- Place the lozenge between your cheeks and gums.
- With the applicator, continuously move Actiq around your mouth, especially over your cheeks. Turn the applicator often.
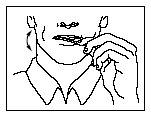
- To make relief more effective, you must finish the Actiq unit completely in about 15 minutes. If you finish it too quickly, you will swallow more medicine and get less relief from breakthrough pain.

- Do not bite or chew the lozenge. This will result in lower blood levels and less relief from breakthrough pain than if used as indicated.
- If for any reason you do not finish the entire lozenge each time you experience breakthrough pain, contact your doctor.
Frequency of Actiq administration
Once you have achieved a dose that effectively controls your pain, do not use more than four units of Actiq per day. If you think you may need more than four units of Actiq per day, you must notify your doctor immediately.
How many units of Actiq to use
Do not use more than two lozenges for a single episode of breakthrough pain.
If you use more Actiq than you should
The most common side effects if you use too much are drowsiness, dizziness, and nausea.
- If you start to feel dizzy, nauseous, or very sleepy before the lozenge has dissolved completely, remove it from your mouth and ask someone else in the household for help.
- A serious side effect of Actiq is slow and/or shallow breathing. This can occur if the dose of Actiq is too high or if you use too much Actiq. In severe cases, taking too much Actiq can also lead to coma. If you feel very dizzy, very sleepy, or have slow or shallow breathing, seek medical help immediately.
- An overdose can also cause a brain disorder known as toxic leukoencephalopathy.
- In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicology Information Service. Phone: 91 562 04 20, indicating the medication and the amount ingested.
What to do if a child or adult accidentally uses Actiq
If you believe someone has accidentally used Actiq, seek medical help immediately. Try to keep the person awake (by calling their name or shaking their arm or shoulder) until medical help arrives.
If you forget to take Actiq
If breakthrough pain still persists, you should use Actiq as your doctor has instructed. If the breakthrough pain disappears, do not use more Actiq until another episode of breakthrough pain appears.
If you stop treatment with Actiq
You should stop Actiq when you no longer have breakthrough pain. However, you should continue taking your usual opioid painkiller for persistent cancer pain, as your doctor has instructed. When you stop treatment with Actiq, you may experience withdrawal symptoms similar to the possible side effects of Actiq. If you experience withdrawal symptoms or are concerned about pain relief, you should consult your doctor. Your doctor will assess whether you need medication to reduce or eliminate withdrawal symptoms.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them. If you notice any side effect, contact your doctor.
Serious side effects
- The most serious side effects are shallow breathing, low blood pressure, and shock.
You or your caregiver should REMOVE the Actiq unit from the mouth, contact your doctor immediately, and seek urgent help if you experience any of the following side effects – you may need urgent medical attention:
- If you are very sleepy or have slow or shallow breathing.
- Difficulty breathing or dizziness, swelling of the tongue, lips, or throat that can be the first signs of a severe allergic reaction.
Note for caregivers:
If you observe that the patient using Actiq has slow and/or shallow breathing or has difficulty waking up, TAKE THE FOLLOWING MEASURES IMMEDIATELY:
- Take the Actiq unit by the applicator, remove it from the patient's mouth, and keep it out of the reach of children or pets until you dispose of it.
- SEEK URGENT ASSISTANCE
- While waiting for urgent assistance, if the person appears to be breathing slowly, encourage them to breathe every 5-10 seconds.
If you feel excessively dizzy, sleepy, or experience any other discomfort while using Actiq, remove the Actiq unit from your mouth using the applicator and dispose of it according to the instructions explained in this leaflet (see section 5). Then, contact your doctor for new instructions on using Actiq.
- Contact your doctor if you experience a combination of the following symptoms
Nausea, vomiting, loss of appetite, fatigue, weakness, dizziness, and low blood pressure.
Together, these symptoms can be a sign of a potentially life-threatening disease called adrenal insufficiency, a disease in which the adrenal glands do not produce enough hormones.
- Prolonged treatment during pregnancy with fentanyl can cause withdrawal syndrome in the newborn, which can be life-threatening (see section 2 Pregnancy and breastfeeding).
Other side effects
Very common:may affect more than 1 in 10 people
- Vomiting, nausea/discomfort, constipation, stomach pain (abdominal)
- Asthenia (weakness), somnolence, sedation, dizziness, headache.
- Shortness of breath.
Common:may affect up to 1 in 10 people
- Confusion, anxiety, seeing or hearing things that are not there (hallucinations), depression, mood changes.
- Feeling unwell
- Muscle spasms, feeling of vertigo or dizziness, loss of consciousness, sedation, feeling of tingling, numbness, difficulty coordinating movements, increased or altered sensitivity to touch, convulsions (seizures).
- Dry mouth, oral inflammation, tongue disorders (e.g., burning sensation or ulcers), taste disturbances.
- Gas, abdominal bloating, indigestion, decreased appetite, weight loss.
- Blurred or double vision.
- Sweating, skin rash, itching.
- Difficulty urinating
- Accidental injuries (e.g., falls).
Uncommon:may affect up to 1 in 100 people
- Dental caries (which can lead to tooth extraction), intestinal paralysis, oral ulcers, gum bleeding.
- Coma, difficulty speaking.
- Abnormal dreams, feeling of indifference, abnormal thoughts, excessive feeling of well-being.
- Vasodilation
- Urticaria
Frequency not known
The following side effects have also been reported with the use of Actiq, but the frequency with which they may occur is unknown:
- Decreased gum, gum inflammation, tooth loss, serious respiratory problems, flushing, feeling of excessive heat, diarrhea, inflammation of arms or legs, fatigue, insomnia, pyrexia, withdrawal syndrome (which can manifest as nausea, vomiting, diarrhea, anxiety, chills, tremors, and sweating).
- Lack of sex hormones (androgen deficiency)
- Drug dependence (addiction) (see section 2)
- Drug abuse (see section 2)
- Pharmacological tolerance (see section 2)
- Delirium (symptoms can include a combination of agitation, restlessness, disorientation, confusion, fear, seeing or hearing things that do not exist, sleep disturbances, nightmares)
- Difficulty breathing during sleep
- Bleeding at the administration site
Prolonged treatment with fentanyl during pregnancy can cause withdrawal symptoms in the newborn, which can be life-threatening (see section 2).
While using Actiq, you may experience irritation, pain, and ulcers at the application site and gum bleeding.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects that do not appear in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Actiq
Keep this medicine in a safe and protected place, where others cannot access it. This medicine can cause serious harm and even be fatal for people who use it accidentally or intentionally when it has not been prescribed for them.
The painkiller in Actiq is very potent and could be potentially fatal for a child if used accidentally. Actiq must be kept out of sight and reach of children.
- Do not use Actiq after the expiration date shown on the blister packaging and carton. The expiration date is the last day of the month indicated.
- Store below 30°C.
- Keep Actiq in its blister packaging until you are ready to use it. Do not use Actiq if the blister packaging is damaged or opened before you are ready to use it.
- If you have stopped using Actiq or have unused units of Actiq at home, return all unused units to your pharmacist.
How to dispose of Actiq once used
Partially used units of Actiq may still contain enough medicine to be harmful or potentially fatal to a child.
Even if there is no medicine left in the applicator, the applicator must be disposed of properly, as follows:
- If there is no medicine left, throw the applicator in a trash can that is out of the reach of children and pets.
- If there is medicine left in the applicator, put the lozenge under a stream of hot water to dissolve the remaining medicine and then throw the applicator in a trash can that is out of the reach of children and pets.
- If you do not finish the entire unit of Actiq and cannot dissolve the remaining medicine immediately, put the Actiq unit out of the reach of children and pets until you have time to dispose of the partially used unit of Actiq as explained.
- Do not flush partially used Actiq units, Actiq applicators, or blister packaging down the toilet.
Medicines should not be disposed of through wastewater or household waste. Deposit the packaging and medicines you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Container Content and Additional Information
Actiq Composition
- The active ingredient is fentanyl. Each lozenge contains:
- 200 micrograms of fentanyl (as citrate)
- 400 micrograms of fentanyl (as citrate)
- 600 micrograms of fentanyl (as citrate)
- 800 micrograms of fentanyl (as citrate)
- 1,200 micrograms of fentanyl (as citrate)
- 1,600 micrograms of fentanyl (as citrate).
- The other components are:
Lozenge:
Hydrated dextrates (equivalent to approximately 1.89 grams of glucose), citric acid, disodium phosphate, artificial berry flavor (maltodextrin (contains glucose), propylene glycol (E1520), artificial flavors, and triethyl citrate), magnesium stearate.
Edible adhesive used to attach the lozenge to the applicator:
Modified cornstarch-based edible starch (E 1450), confectioner's sugar (as sucrose and cornstarch), water.
Printing ink:
Water, desiccated white shellac, synthetic coal tar blue dye (E 133), and ammonium hydroxide to adjust the pH (E527).
Product Appearance and Container Content
Each Actiq lozenge consists of a solid white to off-white medication attached to an oral mucosal applicator. During storage, the lozenge may acquire a slightly speckled appearance. This is due to slight changes in the product's flavoring and does not affect the medication's action in any way.
Actiq is available in 6 different doses: 200, 400, 600, 800, 1,200, and 1,600 micrograms. The dose is marked on the white lozenge, the applicator, the blister pack, and the carton to ensure that you use the correct one. Each dose is associated with a specific color.
Each blister pack contains a single unit of Actiq.
The blisters are supplied in boxes of 3, 6, 15, or 30 individual units of Actiq.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Teva Pharma B.V.
Swensweg 5, 2031 GA Haarlem
Netherlands
Manufacturer
Merckle GmbH
Ludwig-Merckle-Strasse 3
89143 Blaubeuren
Germany
Local Representative
Teva Pharma, S.L.U.
C/ Anabel Segura 11 Edificio Albatros B 1ª planta,
Alcobendas 28108, Madrid (Spain)
Date of the Last Revision of this Prospectus:March 2025
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price140.84 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ACTIQ 1600 micrograms, ORAL LOZENGES WITH INTEGRATED ORAL APPLICATORDosage form: TRANSDERMAL PATCH, 12 MCG/HActive substance: fentanylManufacturer: Aristo Pharma Iberia S.L.Prescription requiredDosage form: BUCCAL/SUCKING TABLET, 1200 microgramsActive substance: fentanylManufacturer: Ferrer Internacional S.A.Prescription requiredDosage form: BUCCAL/SUCKING TABLET, 1600 microgramsActive substance: fentanylManufacturer: Ferrer Internacional S.A.Prescription required
Online doctors for ACTIQ 1600 micrograms, ORAL LOZENGES WITH INTEGRATED ORAL APPLICATOR
Discuss questions about ACTIQ 1600 micrograms, ORAL LOZENGES WITH INTEGRATED ORAL APPLICATOR, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















