

Aurofena

Ask a doctor about a prescription for Aurofena

How to use Aurofena
Leaflet accompanying the packaging: information for the user
AuroFena, 100 micrograms, buccal tablets
AuroFena, 200 micrograms, buccal tablets
AuroFena, 400 micrograms, buccal tablets
Fentanyl
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is AuroFena and what is it used for
- 2. Important information before taking AuroFena
- 3. How to take AuroFena
- 4. Possible side effects
- 5. How to store AuroFena
- 6. Contents of the packaging and other information
1. What is AuroFena and what is it used for
The active substance of AuroFena is fentanyl citrate. AuroFena is a pain-relieving medicine, an opioid used to treat breakthrough pain in adult patients suffering from cancer who are already taking other opioid painkillers to treat persistent (chronic) pain associated with cancer.
Breakthrough pain is additional, sudden pain that occurs despite regular use of opioid painkillers.
2. Important information before taking AuroFena
When NOT to take AuroFena:
- if the patient has not been taking a prescribed opioid medicine (e.g. codeine, fentanyl, hydromorphone, morphine, oxycodone, pethidine) regularly for at least one week to regularly control chronic pain, as planned every day. If the patient has not taken these medicines, they must nottake AuroFena, as this medicine may increase the risk of dangerous release and (or) shallow breathing, and even stop it.
- if the patient is allergic to fentanyl or any of the other ingredients of this medicine (listed in section 6).
- if the patient is taking a medicine containing sodium oxybate.
- if the patient has serious breathing problems or obstructive pulmonary disease.
- if the patient has short-term pain other than breakthrough pain.
Warnings and precautions
Before starting treatment with AuroFena, talk to your doctor or pharmacist.
During treatment with AuroFena, continue to take the opioid painkiller for the treatment of persistent (chronic) pain associated with cancer.
During treatment with AuroFena, do not take other fentanyl-containing medicines previously prescribed for the treatment of breakthrough pain. If you still have these medicines, consult a pharmacist to properly dispose of them.
The medicine should be stored in a safe and protected place where others do not have access to it (more information, see section 5. "How to store AuroFena").
If the patient experiences any of the following symptoms, they should talk to their doctor or pharmacist before starting treatment with AuroFena:
- the dosage of another opioid painkiller used to combat persistent (chronic) pain associated with cancer has not yet been stabilized
- any condition that affects breathing (e.g. asthma, wheezing or shortness of breath)
- head injury
- very slow heartbeat or other heart problems
- liver or kidney problems, as these organs affect how the body processes the medicine
- dehydration or low blood pressure
- age over 65 - the patient may need to use a lower dose, and any dose increase will be closely monitored by the doctor
- heart problems, especially slow heart rate
- the patient is taking benzodiazepines (see section 2 "AuroFena and other medicines"). Taking benzodiazepines may increase the risk of serious side effects, including death.
- the patient is taking antidepressant or antipsychotic medicines (selective serotonin reuptake inhibitors (SSRIs), serotonin and noradrenaline reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs); see section 2 "When not to take AuroFena" and "AuroFena and other medicines"). Taking these medicines with AuroFena may lead to a potentially life-threatening condition called serotonin syndrome (see section 2 "AuroFena and other medicines").
- the patient has ever had adrenal insufficiency, a condition in which the adrenal glands do not produce enough hormones or a lack of sex hormones (androgen deficiency) while taking opioids (see section 4 "Serious side effects")
- the patient has ever abused or been addicted to opioids or any other medicine, alcohol, or drugs
- alcohol consumption; see section "Taking AuroFena with food, drink, and alcohol".
Talk to your doctor if, DURINGtreatment with AuroFena:
- the patient feels pain or increased sensitivity to pain (hyperalgesia) that does not respond to a higher dose of painkiller prescribed by the doctor.
- the patient experiences several of the following symptoms: nausea, vomiting, loss of appetite, fatigue, weakness, dizziness, and low blood pressure. These symptoms may be a sign of a potentially life-threatening condition called adrenal insufficiency, in which the adrenal glands do not produce enough hormones.
- the patient has breathing disorders during sleep: AuroFena may cause sleep-related breathing disorders, such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen levels in the blood). Symptoms may include pauses in breathing during sleep, nighttime awakenings due to shortness of breath, difficulty staying asleep, or excessive daytime sleepiness. If the patient or another person notices these symptoms, they should contact their doctor. The doctor may consider reducing the dose.
Long-term use and tolerance
This medicine contains fentanyl, which is an opioid. Repeated use of opioid painkillers can lead to reduced efficacy of the medicine (the patient gets used to it, which is known as tolerance to the medicine). During treatment with AuroFena, the patient's sensitivity to pain may also increase. This phenomenon is known as hyperalgesia.
Increasing the dose of AuroFena may temporarily reduce the intensity of the pain, but it can also be harmful. If the patient notices a decrease in the efficacy of the medicine, they should consult their doctor. The doctor will decide whether it is better for the patient to increase the dose or gradually reduce the use of AuroFena.
Dependence and addictive use
Repeated use of AuroFena can also lead to dependence, abuse, and addictive use, which can result in life-threatening overdose.
The risk of these side effects may increase with increasing dose and duration of use. Dependence or addictive use can cause the patient to feel a loss of control over how much medicine to use or how often to take it. The patient may feel the need to continue using the medicine, even if it does not help alleviate their pain.
The risk of dependence on AuroFena or its addictive use varies from person to person. The risk of dependence on AuroFena or its addictive use may be higher if:
- the patient or anyone in their family has ever abused or been addicted to alcohol, prescription medicines, or illegal substances ("addiction");
- the patient smokes tobacco;
- the patient has ever had mood disorders (depression, anxiety disorders, or personality disorders) or has been treated by a psychiatrist for other mental illnesses.
If the patient experiences any of the following symptoms while taking AuroFena, it may indicate dependence or addictive use.
- The patient must take the medicine for a longer period than prescribed by the doctor.
- The patient must take a higher dose than recommended.
- The patient uses the medicine for reasons other than those for which the doctor prescribed it, such as "to calm down" or "to fall asleep".
- The patient has repeatedly tried to stop or control the use of the medicine but failed.
- After stopping the medicine, the patient feels unwell (e.g. they experience nausea, vomiting, diarrhea, anxiety, tremors, seizures, and excessive sweating), and they feel better when they take the medicine again ("withdrawal effect").
If the patient notices any of these symptoms, they should discuss the best treatment strategy with their doctor, including determining when it is appropriate to stop treatment and how to safely stop treatment.
Seek IMMEDIATEmedical attention if:
- While taking AuroFena, the patient experiences symptoms such as difficulty breathing or dizziness, swelling of the tongue, lips, or throat. These may be early signs of a serious allergic reaction (anaphylaxis, hypersensitivity; see section 4 "Serious side effects").
| This medicine contains fentanyl, which is an opioid. It can cause dependence and (or) addiction. | |
Accidental ingestion of AuroFena
If it is suspected that someone has accidentally taken AuroFena, seek medical attention immediately. Try to keep the person awake until the ambulance arrives.
The person who has accidentally taken AuroFena may experience the same side effects as described in the section "Taking a higher dose of AuroFena than recommended".
Children and adolescents
Do not give this medicine to children and adolescents under 18 years of age.
AuroFena and other medicines
Before starting treatment with AuroFena, tell your doctor or pharmacist if you are currently taking, have recently taken, or plan to take any of the following medicines:
- concomitant use of fentanyl and sedative medicines, such as benzodiazepines or similar medicines, increases the risk of drowsiness, breathing difficulties (respiratory depression), coma, and can be life-threatening. For this reason, concomitant use can only be considered if other treatment options are not possible. Nevertheless, if the doctor prescribes fentanyl with sedative medicines, the doctor will limit the dose and duration of concomitant treatment. You should inform your doctor about all sedative medicines you are currently taking (such as sleeping pills, anti-anxiety medicines, certain medicines used to treat allergic reactions (antihistamines) or calming medicines) and strictly follow the dose recommended by the doctor. It may be helpful to inform friends and relatives and make them aware of the above symptoms. If such symptoms occur, contact your doctor.
Concomitant use of medicines containing sodium oxybate and fentanyl is contraindicated (see "When not to take AuroFena"). Treatment with sodium oxybate should be discontinued before starting treatment with AuroFena.
- Certain muscle relaxants, such as baclofen, diazepam (see also section "Warnings and precautions").
- Medicines that may affect how the body processes fentanyl, such as ritonavir, nelfinavir, amprenavir, and fosamprenavir (medicines used to control HIV infection) or other so-called CYP3A4 inhibitors, such as ketoconazole, itraconazole, or fluconazole (for the treatment of fungal infections), troleandomycin, clarithromycin, or erythromycin (for the treatment of bacterial infections), aprepitant (for the treatment of severe nausea) and diltiazem and verapamil (for the treatment of high blood pressure and heart disease).
- Medicines called monoamine oxidase inhibitors (MAOIs) (used in severe depression), or have been taken within the last 2 weeks.
- Certain strong painkillers, called partial agonists/antagonists, e.g. buprenorphine, nalbuphine, and pentazocine (painkillers). When taking these medicines, the patient may experience withdrawal symptoms (nausea, vomiting, diarrhea, anxiety, tremors, and sweating).
- Certain medicines used to treat neuropathic pain (gabapentin and pregabalin).
- The risk of side effects increases if the patient is taking certain antidepressant or antipsychotic medicines. AuroFena may interact with these medicines, and the patient may experience changes in mental state (e.g. agitation, hallucinations, coma) and other effects, such as body temperature above 38°C, increased heart rate, unstable blood pressure, and increased reflexes, muscle stiffness, lack of coordination, and (or) gastrointestinal symptoms (e.g. nausea, vomiting, diarrhea). The doctor will tell the patient if AuroFena is suitable for them.
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
Taking AuroFena with food, drink, and alcohol
- AuroFena can be taken before or after, but not during, a meal. Before taking AuroFena, you can drink a small amount of water to moisten your mouth, but do not drink or eat during treatment.
- Do not drink grapefruit juice while taking AuroFena, as it may affect how the body processes fentanyl.
- Do not drink alcohol while taking AuroFena, as it may increase the risk of serious side effects, including death.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before taking this medicine.
Pregnancy
AuroFena should not be used in pregnant women unless prescribed by a doctor. If AuroFena is used for a long time during pregnancy, there is also a risk of withdrawal symptoms in the newborn, which, if not recognized and treated by a doctor, can be life-threatening.
Do not use AuroFena during labor, as fentanyl may cause respiratory depression in the newborn.
Breastfeeding
Fentanyl may pass into breast milk and cause side effects in the breastfed child.
Do not use AuroFena while breastfeeding. Do not start breastfeeding until at least 5 days after the last dose of AuroFena.
Driving and using machines
Ask your doctor if you can safely drive or operate machinery after taking AuroFena. Do not drive or operate machinery if you experience drowsiness or dizziness, blurred or double vision, or difficulty concentrating. It is essential to know how you react to AuroFena before driving or operating machinery.
AuroFena contains sorbitol
This medicine contains 67.1 mg of sorbitol in each buccal tablet.
3. How to take AuroFena
Always take this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
Before starting and regularly during treatment, your doctor will also discuss with you what to expect from taking AuroFena, when and for how long to take it, when to see a doctor, and when to stop taking the medicine (see also section 2).
Dose and frequency of administration
When starting treatment with AuroFena, the doctor will, in agreement with the patient, choose a suitable dose to relieve breakthrough pain. AuroFena should always be taken exactly as the doctor has prescribed. The initial dose is 100 micrograms. During dose adjustment, the doctor may recommend taking more than one tablet at a time during an episode. If breakthrough pain does not subside after 30 minutes, only 1 additional tablet of AuroFena should be taken during dose adjustment.
After the doctor has determined the suitable dose, the general rule is to take 1 tablet at a time during an episode of breakthrough pain. In the future, pain treatment may need to be changed. Higher doses may be necessary. If breakthrough pain does not subside after 30 minutes, only 1 additional tablet of AuroFena can be taken during repeated dose adjustment.
If the chosen dose of AuroFena is not sufficient to relieve breakthrough pain, the patient should contact their doctor, who will decide whether the dose should be changed.
Wait at least 4 hours before taking AuroFena to treat another episode of breakthrough pain.
Immediately inform your doctor if you are taking AuroFena more than 4 times a day, as the treatment plan may need to be changed. The doctor may change the way chronic pain is treated. When better control over persistent pain is achieved, the doctor may change the dose of AuroFena. If the doctor suspects increased sensitivity to pain (hyperalgesia) associated with fentanyl use, they may consider reducing the fentanyl dose (see section 2 "Warnings and precautions"). To achieve effective treatment, the patient should inform the doctor about the pain experienced and the effects of taking AuroFena, so that the dose can be modified if necessary.
Do not change the dose of AuroFena or other painkillers on your own. Dose changes must be prescribed and monitored by a doctor.
If you have any questions about taking the medicine or doubts about the correct dose, consult your doctor again.
Method of administration
AuroFena buccal tablets are taken on the mucous membrane of the mouth.
After placing the tablet in the mouth, it dissolves, and the medicine is absorbed into the bloodstream through the mucous membrane of the mouth. Taking the medicine in this way allows for its rapid absorption and relief of breakthrough pain.
Taking the medicine
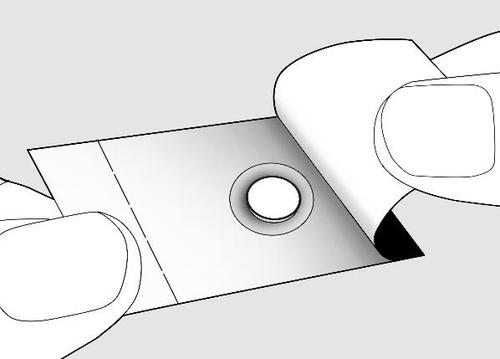
- Open the blister only when you are ready to take the tablet. The tablet must be taken immediately after removal from the blister.
- Separate the single-dose unit from the rest of the blister by tearing along the perforation line.
- Bend the single-dose unit along the marked line.
- Remove the foil from the back of the blister to expose the tablet. Do nottry to push the tablet through the blister, as it may be damaged.
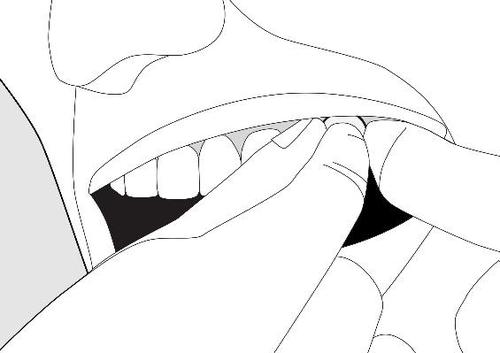
- Remove the tablet from the blister and immediatelyplace the tablet near the molar tooth, between the gum and cheek (as shown in the picture). Sometimes the doctor may recommend placing the tablet under the tongue.
- Do not try to crush or divide the tablet.
- Do not chew, suck, chew, or swallow the tablet, as this will result in less pain relief than taking the tablet as recommended.
- The tablet should remain between the upper gum and cheek until it dissolves. This may take up to 30 minutes. If irritation occurs, the position of the tablet on the gum can be changed.
- After 30 minutes, if tablet residues remain in the mouth, they can be swallowed with a glass of water.
Taking a higher dose of AuroFena than recommended
- The most common side effects are drowsiness, nausea, or dizziness. If the patient feels strong dizziness or excessive drowsiness before the tablet has completely dissolved, they should immediately rinse their mouth with water and spit out the remaining tablet into the sink or toilet.
- A serious side effect of fentanyl is slow and (or) shallow breathing. This can happen if the dose of fentanyl is too high or if the patient takes AuroFena in too large quantities. In severe cases, symptoms of taking too much AuroFena can lead to coma. If dizziness, excessive drowsiness, or slow and (or) shallow breathing occur, seek medical attention immediately.
- Overdose can also cause brain disorders called toxic leukoencephalopathy.
Missing a dose of AuroFena
If breakthrough pain is still present, take AuroFena as recommended by your doctor.
If the breakthrough pain has subsided, do not take AuroFena until the next episode of breakthrough pain occurs.
Stopping treatment with AuroFena
Stop taking AuroFena when you no longer have breakthrough pain. However, continue to take your regular opioid painkiller for the treatment of persistent (chronic) pain associated with cancer as recommended by your doctor. When stopping fentanyl, withdrawal symptoms may occur, similar to possible side effects of AuroFena. If withdrawal symptoms occur or if you are concerned about pain relief, consult your doctor. The doctor will assess whether you need a medicine to reduce or eliminate withdrawal symptoms.
If you have any further doubts about taking this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, AuroFena can cause side effects, although not everybody gets them.
If you experience any side effects, talk to your doctor or pharmacist.
Serious side effects
- The most serious side effects are slow and (or) shallow breathing, low blood pressure, and shock.
AuroFena, like other fentanyl-containing medicines, can cause very severe breathing difficulties, which can lead to death. If excessive drowsiness and (or) slow and (or) shallow breathing occur, the patient or their caregiver should immediately contact a doctor and
call emergency services.
- Seek medical attention immediately if the patient experiences any of the following symptoms
- Nausea, vomiting, loss of appetite, fatigue, weakness, dizziness, and low blood pressure.
The occurrence of these symptoms at the same time may be a sign of a potentially life-threatening condition called adrenal insufficiency, in which the adrenal glands do not produce enough hormones.
Other side effects
Very common:may affect more than 1 in 10 people
- dizziness, headache
- feeling of nausea, vomiting
- at the site of tablet placement: pain, ulceration, irritation, bleeding, numbness, loss of sensation, redness, swelling, or abscess Common:may affect up to 1 in 10 people
- feeling of anxiety or confusion, depression, insomnia
- taste disorders, weight loss
- drowsiness, calmness, excessive fatigue, weakness, migraine, numbness, swelling of hands or feet, withdrawal syndrome (may manifest with the following side effects: nausea, vomiting, diarrhea, anxiety, tremors, and sweating), tremors, falls, chills
- constipation, oral inflammation, dry mouth, diarrhea, heartburn, loss of appetite, stomach pain, gastrointestinal disorders, nausea
- itching, excessive sweating, rash
- shortness of breath, sore throat
- decreased white blood cell count, decreased red blood cell count, low or high blood pressure, rapid heart rate
- muscle pain, back pain
- fatigue
Uncommon:may affect up to 1 in 100 people
- sore throat
- decreased platelet count
- euphoric mood, nervousness, feeling of abnormality, tremors or slowing; seeing or hearing things that do not exist (hallucinations), decreased consciousness, psychiatric changes, dependence on the medicine (addiction), disorientation, lack of concentration, loss of balance, dizziness, speech disorders, ringing in the ears, ear disorders
- blurred or blurred vision, red eyes
- slow heart rate, feeling of intense heat (hot flashes)
- severe breathing difficulties, sleep apnea
- one or more of the following oral disorders: ulceration, loss of sensation, discomfort, discoloration, soft tissue disorders, tongue disorders, pain, blisters, or ulceration of the tongue, gum pain, dry mouth, oral disorders
- esophageal inflammation, intestinal obstruction, biliary disorders
- cold sweats, facial swelling, general itching, hair loss, muscle tremors, muscle weakness, malaise, chest discomfort, thirst, feeling of cold or heat, difficulty urinating
- malaise
- sudden flushing of the face
Rare:may affect up to 1 in 1,000 people
- thought disorders, movement disorders
- blistering in the mouth, dry mouth, accumulation of pus in the oral mucosa
- testosterone deficiency, abnormal eye sensation, sensation of seeing flashes, brittle nails
- allergic reactions, such as rash, flushing, swelling of the lips and face, hives
Unknown:frequency cannot be estimated from the available data
- loss of consciousness, respiratory arrest, seizures (fits)
- hormone deficiency (androgen deficiency)
- dependence on medicines (addiction) (see section 2)
- abuse of medicines (see section 2)
- tolerance to the medicine (see section 2)
- delirium (may occur with several of the following symptoms: agitation, restlessness, disorientation, confusion, anxiety, seeing or hearing things that do not exist, sleep disturbances, nightmares)
- long-term treatment with fentanyl during pregnancy may cause withdrawal syndrome in the newborn, which can be life-threatening (see section 2)
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, Al. Jerozolimskie 181C, 02-222 Warszawa, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, you can help provide more information on the safety of this medicine.
Side effects can also be reported to the marketing authorization holder.
5. How to store AuroFena
Keep the medicine in a safe and protected place where others do not have access to it. It can cause serious harm and lead to death if taken accidentally or intentionally by someone for whom it was not prescribed.
The pain-relieving substance in AuroFena is very strong and can be life-threatening if taken accidentally by a child. This medicine must be kept out of sight and out of reach of children.
- Do not use this medicine after the expiry date stated on the carton and blister after "EXP". The expiry date refers to the last day of that month.
- Do not store above 30°C. Store in the original packaging to protect from moisture.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What AuroFena contains
The active substance is fentanyl. Each tablet contains:
- 100 micrograms of fentanyl (as citrate)
- 200 micrograms of fentanyl (as citrate)
- 400 micrograms of fentanyl (as citrate) The other ingredients are mannitol, sorbitol, citric acid, macrogol 6000, L-arginine, magnesium stearate.
What AuroFena looks like and contents of the pack
The buccal tablets are white, round with beveled edges, with the number "1" embossed on one side for AuroFena 100 micrograms, "2" for AuroFena 200 micrograms, "4" for AuroFena 400 micrograms. Each tablet is approximately 10 mm in diameter.
Each blister contains 4 buccal tablets, available in cartons containing 4 or 28 buccal tablets.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Aurovitas Pharma Polska Sp. z o.o.
ul. Sokratesa 13D lokal 27
01-909 Warszawa
Manufacturer:
BLUEPHARMA – INDÚSTRIA FARMACÊUTICA, S.A.
- S. Martinho do Bispo 3045-016 Coimbra Portugal
Ardena Pamplona, S.L.
Calle Noáin 1
31110 Noáin, Navarra
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Netherlands
Fentanyl Aurobindo 100, 200, 400, 600, 800 mcg muco-adhesive buccal tablets
Poland
AuroFena
Date of last revision of the leaflet: 04.2025
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterArdena Pamplona S.L. Bluepharma Indústria Farmacêutica, S.A
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AurofenaDosage form: Tablets, 200 mcgActive substance: fentanylPrescription requiredDosage form: System, 25 mcg/hActive substance: fentanylManufacturer: Janssen Pharmaceutica N.V.Prescription required
Alternatives to Aurofena in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Aurofena in Espanha
Alternative to Aurofena in Ukraine
Online doctors for Aurofena
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Aurofena – subject to medical assessment and local rules.














