
CHENODEOXYCHOLIC ACID LEADIANT 250 mg HARD CAPSULES


How to use CHENODEOXYCHOLIC ACID LEADIANT 250 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Chenodeoxycholic Acid Leadiant 250 mg Hard Capsules
Chenodeoxycholic Acid
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because
it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist, even if you think they are not serious. See section 4.
Contents of the Package Leaflet
- What is Chenodeoxycholic Acid Leadiant and what is it used for
- What you need to know before you take Chenodeoxycholic Acid Leadiant
- How to take Chenodeoxycholic Acid Leadiant
- Possible side effects
- Storage of Chenodeoxycholic Acid Leadiant
- Contents of the pack and other information
1. What is Chenodeoxycholic Acid Leadiant and what is it used for
Chenodeoxycholic Acid Leadiant capsules contain a substance called chenodeoxycholic acid. This substance is normally produced in the liver from cholesterol. It is part of bile, a liquid that helps digest fats and vitamins from food. Patients with a rare disease called cerebrotendinous xanthomatosis (CTX) cannot produce chenodeoxycholic acid, which causes an accumulation of fatty acid deposits in various parts of the body. This accumulation can cause damage to the affected areas.
Chenodeoxycholic Acid Leadiant capsules treat CTX by replacing chenodeoxycholic acid, thus preventing the accumulation of fatty acid deposits.
Chenodeoxycholic Acid Leadiant capsules can be used from the first month of life and patients with CTX require treatment for the rest of their life.
2. What you need to know before you take Chenodeoxycholic Acid Leadiant
Do not take Chenodeoxycholic Acid Leadiant
- if you are allergic to chenodeoxycholic acid or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Chenodeoxycholic Acid Leadiant should be used under medical supervision. During treatment, your doctor will perform blood and urine tests to monitor your response to this medicine and to adjust the dose if necessary. Your doctor will inform you if there is any reason why you should stop treatment with Chenodeoxycholic Acid Leadiant.
Infants
The safety and efficacy of Chenodeoxycholic Acid Leadiant in infants under one month of age have not been studied.
Taking Chenodeoxycholic Acid Leadiant with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
The following medicines may affect Chenodeoxycholic Acid Leadiant levels:
- ciclosporin and sirolimus (medicines used to suppress the immune system)
- phenobarbital (a medicine for epilepsy)
If your doctor considers it necessary for you to take ciclosporin, sirolimus or phenobarbital, they will closely monitor your blood and urine test results and adjust the dose of Chenodeoxycholic Acid Leadiant if necessary.
Oral contraceptives may affect the functioning of Chenodeoxycholic Acid Leadiant, making it less effective. It is not recommended to take oral contraceptives during treatment with Chenodeoxycholic Acid Leadiant. Consult your doctor about other suitable contraceptive methods.
The following medicines may reduce the effect of Chenodeoxycholic Acid Leadiant:
- cholestyramine, colestipol (bile acid sequestrants)
- medicines for heartburn (antacids) containing aluminium hydroxide and/or smectite (aluminium oxide)
If you need to take colestyramine, take Chenodeoxycholic Acid Leadiant one hour before colestyramine or 4 to 6 hours after.
Other medicines mentioned should be taken 2 hours before or 2 hours after taking Chenodeoxycholic Acid Leadiant.
Consult your doctor if you are taking any of these medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Chenodeoxycholic Acid Leadiant is not recommended during pregnancy and breastfeeding. It is not known whether Chenodeoxycholic Acid Leadiant passes into breast milk.
Women should use an effective contraceptive method while being treated with Chenodeoxycholic Acid Leadiant. Oral contraceptives are not recommended. Consult your doctor about other suitable contraceptive methods.
Driving and using machines
Chenodeoxycholic Acid Leadiant is not expected to affect your ability to drive or use machines.
3. How to take Chenodeoxycholic Acid Leadiant
Follow exactly the administration instructions of this medicine given by your doctor.
In case of doubt, consult your doctor again. The usual initial dose is one 250 mg capsule three times a day.
The maximum dose is one 250 mg capsule three times a day. The maximum dose is one 250 mg capsule four times a day. The capsules should be taken whole and with water, approximately at the same time every day. The capsules can be taken with or without food. Your doctor may decide to increase your dose depending on how your body responds to treatment. Your doctor will tell you how many capsules you need to take and when you should take them.
Use in children and adolescents (from 1 month to 18 years)
In infants and children, the dose should be calculated based on body weight. The initial dose will be 5 mg per kg per day. The maximum dose for children is 15 mg per kg per day. Your doctor will decide on the number and frequency of doses to cover the child's total daily dose. Your doctor may decide to change the dose depending on how the child's body responds to treatment.
For infants, children, and adults who cannot swallow capsules and/or need to take a dose less than 250 mg, these can be opened and mixed with a sodium bicarbonate solution (8.4%, 1 mmol/ml) to produce a suspension containing 10 mg/ml of chenodeoxycholic acid.
For infants: the capsules can be opened to add their contents to 50 ml of sodium bicarbonate solution (8.4%, 1 mmol/ml) to produce a suspension containing 5 mg/ml of chenodeoxycholic acid.
Remove the mixture until all the powder is suspended. Make sure to introduce all the powder that has remained on the edges into the mixing glass and stir the contents (for about 5 minutes) until the mixture is homogeneous without visible powder residues. The mixture will be ready when no powder residues are visible.
The resulting suspension contains 22.9 mg of sodium per ml, which should be taken into account in patients with low-sodium diets.
The suspension is easily formed and is ready when no powder residues are visible. It is recommended that this suspension be prepared in a pharmacy and that instructions be provided to parents on how to administer it.
The suspension should be stored in a glass container. Do not refrigerate or freeze. The suspension remains stable for a maximum of 7 days.
In the pharmacy, you can be provided with oral dosing syringes with the correct volume and graduation to administer the suspension. It is preferable that oral syringes have the correct volumes marked.
A label from the pharmacy should be placed on the container, including the patient's name, dosing instructions, expiration date, medicine name, and any other information required by local pharmacy regulations.
Your doctor will provide information on the dose to be administered to the child based on their weight. The dosing interval in children is 5-15 mg/kg per day.
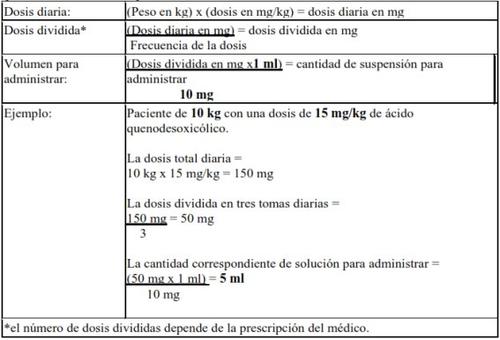
Dose calculation (children from 1 to 12 years, adolescents from 12 to 18 years, and adults):10 mg/mlof chenodeoxycholic acid in suspension
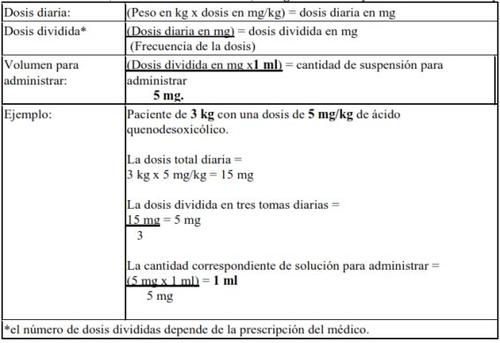
Dose calculation (infants from 1 month to 1 year):5 mg/mlof chenodeoxycholic acid in suspension
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Adverse reactions of unknown frequency (cannot be estimated from the available data)
- Constipation
- Abnormal liver function test values
Reporting of side effects
If you experience side effects, talk to your doctor or pharmacist, even if you think they are not serious. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Chenodeoxycholic Acid Leadiant
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the blisters after “EXP”. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
- What Chenodeoxycholic Acid Leadiant contains
- The active substance is chenodeoxycholic acid.
- Each capsule contains 250 mg of chenodeoxycholic acid.
- The other ingredients are:
Each capsule contains: corn starch, magnesium stearate, colloidal anhydrous silica, water. Capsule shell: gelatin, titanium dioxide (E 171), quinoline yellow (E 104), erythrosine (E 127)
Appearance of the product and pack contents
Chenodeoxycholic Acid Leadiant is presented in hard capsules. The capsules are formed by a yellow body and an orange cap containing a white compressed powder.
Chenodeoxycholic Acid Leadiant is available in blisters containing 100 hard capsules.
Marketing authorisation holder
Leadiant GmbH
Liebherrstr. 22
80538 Munich
Germany
Email: [email protected]
Manufacturer
Pharmaloop S.L.
C/Bolivia, no 15 Polígono Industrial Azque
Alcalá de Henares
Madrid 28806
Spain
Date of last revision of this leaflet: <{MM/AAAA}>.
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
--------------------------------------------------------------------------------------------------------------------
Information for healthcare professionals
Patients who cannot swallow the capsules
For children, adolescents, and adults who cannot swallow the capsules, or who need to take doses less than 250 mg, these can be opened to add their contents to 25 ml of sodium bicarbonate solution (8.4%, 1 mmol/ml) to produce a suspension containing 10 mg/ml of chenodeoxycholic acid.
For infants: the capsules can be opened to add their contents to 50 ml of sodium bicarbonate solution (8.4%, 1 mmol/ml) to produce a suspension containing 5 mg/ml of chenodeoxycholic acid.
Remove the mixture until all the powder is suspended. Make sure to introduce all the powder that has remained on the edges into the mixing glass and stir the contents (for about 5 minutes) until the mixture is homogeneous without visible powder residues. The mixture will be ready when no powder residues are visible.
The resulting suspension contains 22.9 mg of sodium per ml, which should be taken into account in patients with low-sodium diets.
The suspension is easily formed and is ready when no powder residues are visible. It is recommended that this suspension be prepared in a pharmacy and that instructions be provided to parents on how to administer it.
The suspension should be stored in a glass container. Do not refrigerate or freeze. The suspension remains stable for a maximum of 7 days.
In the pharmacy, you can be provided with oral dosing syringes with the correct volume and graduation to administer the suspension. It is preferable that oral syringes have the correct volumes marked.
A label from the pharmacy should be placed on the container, including the patient's name, dosing instructions, expiration date, medicine name, and any other information required by local pharmacy regulations.
Your doctor will provide information on the dose to be administered to the child based on their weight. The dosing interval in children is 5-15 mg/kg per day.
Dose calculation (children from 1 to 12 years, adolescents from 12 to 18 years, and adults):10 mg/mlof chenodeoxycholic acid in suspension
Dose calculation (infants from 1 month to 1 year):5 mg/mlof chenodeoxycholic acid in suspension
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CHENODEOXYCHOLIC ACID LEADIANT 250 mg HARD CAPSULESDosage form: CAPSULE, 250 mgActive substance: ursodeoxycholic acidManufacturer: Grindeks AsPrescription requiredDosage form: TABLET, 150 mgActive substance: ursodeoxycholic acidManufacturer: Glenmark Arzneimittel GmbhPrescription requiredDosage form: CAPSULE, 250 mgActive substance: ursodeoxycholic acidManufacturer: Glenmark Arzneimittel GmbhPrescription required
Online doctors for CHENODEOXYCHOLIC ACID LEADIANT 250 mg HARD CAPSULES
Discuss questions about CHENODEOXYCHOLIC ACID LEADIANT 250 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















