
ACICLOVIR BRILL PHARMA 30 mg/g OPHTHALMIC OINTMENT

How to use ACICLOVIR BRILL PHARMA 30 mg/g OPHTHALMIC OINTMENT
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Aciclovir Brill Pharma 30 mg/g Ophthalmic Ointment
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Aciclovir Brill Pharma and what is it used for
- What you need to know before you use Aciclovir Brill Pharma
- How to use Aciclovir Brill Pharma
- Possible side effects
- Storing Aciclovir Brill Pharma
- Contents of the pack and other information
1. What is Aciclovir Brill Pharma and what is it used for
This medicine contains aciclovir, an active substance belonging to a group of medicines called antivirals.
It is indicated for the treatment of keratitis (inflammation of the cornea) caused by the herpes simplex virus.
2. What you need to know before you use Aciclovir Brill Pharma
Do not use Aciclovir Brill Pharma
If you are allergic to aciclovir, valaciclovir, or any of the other ingredients of this medicine (listed in section 6).
If you think this applies to you, do not use this medicine until you have consulted your doctor.
Warnings and precautions
Consult your doctor or pharmacist before you start using this medicine.
A mild and transient stinging sensation may occur immediately after applying this medicine.
Be careful with this medicine. Before using Aciclovir Brill Pharma, your doctor should know if you wear contact lenses. Stop wearing contact lenses during treatment with this medicine. If you are not sure, consult your doctor or pharmacist before using this medicine.
Other medicines and Aciclovir Brill Pharma
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
Although no interactions of this medicine have been described through ocular administration, consult your doctor before using other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
Your doctor will assess the benefit to you and the risk to your baby of using this medicine during pregnancy.
Breastfeeding
Although after topical administration of this medicine, systemic absorption is minimal, your doctor will assess the benefit to you and the risk to your baby of using this medicine during breastfeeding.
Driving and using machines
You may notice blurred vision immediately after applying this medicine. Do not drive or use machines until this effect has disappeared.
3. How to use Aciclovir Brill Pharma
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
Adults and children:
- Apply approximately 10 mm of Aciclovir Brill Pharma to the infected eye 5 times a day.
- This medicine should be applied at 4-hour intervals. The suggested times are: 7 am, 11 am, 3 pm, 7 pm, and 11 pm.
- Your vision may be blurred for 5 or 10 minutes after applying this medicine.
- Treatment should continue for at least 3 days after the eye has shown improvement.
If you are using more than one medicine via the ocular route, you should space the applications of the medicines at least 5 minutes apart or as indicated by your doctor. Ophthalmic ointments should be administered last.
How to apply Aciclovir Brill Pharma to the eye
This medicine should be administered only via the ocular route.
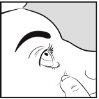
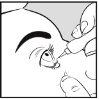

- Wash your hands
- Use your finger to gently pull down the lower eyelid of the infected eye
- Lean your head slightly back and look up
- Apply 10 mm of Aciclovir Brill Pharma to the inside of the lower eyelid. Try to avoid the tip of the tube touching any part of the eye.
- Keep the eye closed for 30 seconds.
- Wash your hands after applying the ointment.
If you use more Aciclovir Brill Pharma than you should
If you use more Aciclovir Brill Pharma than you should, carefully remove the excess. It is unlikely that an overdose will occur with this medicine.
However, no adverse effects are expected in the event of ingesting the 135 mg of aciclovir contained in the package.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Aciclovir Brill Pharma
If you forget an application, do it as soon as possible and continue the treatment as prescribed. However, if it is close to the time of your next application, skip the missed application.
Do not use a double dose to make up for missed doses.
If you have any other questions about using this medicine, ask your doctor or pharmacist.
If you stop using Aciclovir Brill Pharma
Do not stop using Aciclovir Brill Pharma without consulting your doctor.
It is important that you use this medicine for the indicated time to completely eliminate the infection.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common side effects (may affect more than 1 in 10people):
- Irritation or foreign body sensation in the eye (feeling of having something in the eye)
- Effects on the surface of the cornea (transparent layer in front of the iris and pupil) that can cause changes in vision (blurred vision) and/or uncomfortable sensitivity to light.
It does not require interruption of treatment and heals without apparent sequelae.
Common side effects (may affect up to 1 in 10people):
- Mild and transient stinging sensation in the eye immediately after application.
- Red, watery, and itchy eyes (conjunctivitis).
Rare side effects (may affect up to 1 in 1,000people):
- Inflammation of the eyelid (blepharitis).
Very rare side effects (may affect up to 1 in 10,000people):
- Immediate hypersensitivity reactions (allergic reactions).
The signs of allergic reactions may include:
- swelling of the face, lips, tongue, or other parts of the body (angioedema).
- rash, itching, or hives on the skin.
- shortness of breath, difficulty breathing, or wheezing.
- unexplained fever and feeling of fainting, especially while standing.
Contact a doctor immediately if you have any of these symptoms. Stop using Aciclovir Brill Pharma.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Health Products Agency (AEMPS) website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Aciclovir Brill Pharma
Keep out of sight and reach of children.
Store below 25°C.
Do not use this medicine after the expiry date stated on the packaging after "EXP". The expiry date is the last day of the month indicated.
Discard 4 weeks after first opening.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the SIGRE collection point at your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Contents of the pack and other information
Composition of Aciclovir Brill Pharma
- The active substance is aciclovir. Each gram of ointment contains 30 mg of aciclovir.
- The other ingredients (excipients) are: white petrolatum.
Appearance and packaging of the product
Tube (aluminum) with screw cap (polyethylene) packaged in cardboard boxes. Each box contains a 4.5-gram tube of ointment.
Marketing authorization holder and manufacturer
Marketing authorization holder
BRILL PHARMA, S.L.
C/ Munner, 8
08022 Barcelona
Spain
Manufacturer
URSAPHARM Arzneimittel GmbHIndustriestraße 35
66129 Saarbrücken
Germany
Date of the last revision of this leaflet: June 2020
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price15.06 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ACICLOVIR BRILL PHARMA 30 mg/g OPHTHALMIC OINTMENTDosage form: OPHTHALMIC OINTMENT, 30 mg/gActive substance: aciclovirManufacturer: Agepha Pharma S.R.O.Prescription requiredDosage form: OPHTHALMIC GEL, 0.15%Active substance: ganciclovirManufacturer: Laboratoires TheaPrescription requiredDosage form: EYEDROP, 5 mg/mlActive substance: moxifloxacinManufacturer: Tiedra Farmaceutica S.L.Prescription required
Online doctors for ACICLOVIR BRILL PHARMA 30 mg/g OPHTHALMIC OINTMENT
Discuss questions about ACICLOVIR BRILL PHARMA 30 mg/g OPHTHALMIC OINTMENT, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















