

Xilometazolin Teva

Ask a doctor about a prescription for Xilometazolin Teva

How to use Xilometazolin Teva
Package Leaflet: Information for the User
Xylometazolin Teva, 1 mg/ml, Nasal Spray, Solution
Xylometazolini hydrochloridum
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet for the patient or as directed by a doctor or pharmacist.
- This package leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the package leaflet, the doctor or pharmacist should be informed. See section 4.
- If there is no improvement after 7 days or the patient feels worse, a doctor should be consulted.
Package Leaflet Contents:
- 1. What Xylometazolin Teva is and what it is used for
- 2. Important information before using Xylometazolin Teva
- 3. How to use Xylometazolin Teva
- 4. Possible side effects
- 5. How to store Xylometazolin Teva
- 6. Contents of the package and other information
1. What Xylometazolin Teva is and what it is used for
If after 7 days of using the medicine there is no improvement or the patient feels worse, a doctor should be consulted.
Xylometazolin Teva contains the active substance xylometazoline. It is a decongestant with local action, which reduces the swelling of the nasal mucosa and relieves the feeling of a "stuffy" nose. The medicine reduces the swelling of the nasal mucosa.
Xylometazolin Teva is used for the short-term treatment of nasal congestion symptoms caused by rhinitis or sinusitis.
Xylometazolin Teva 1 mg/ml is intended for adults and children aged 10 and over.
2. Important information before using Xylometazolin Teva
When not to use Xylometazolin Teva
- if the patient is allergic to xylometazoline hydrochloride or any of the other ingredients of the medicine (listed in section 6),
- if the patient has recently undergone neurosurgical procedures (transsphenoidal removal of the pituitary gland or other surgery involving exposure of the dura mater),
- if the patient suffers from atrophic rhinitis (a form of chronic inflammation of the nasal mucosa that causes dryness and crusting of the nasal mucosa),
- in children under 10 years of age.
Warnings and precautions
Before starting to use Xylometazolin Teva, the patient should discuss it with a doctor or pharmacist:
- if the patient is taking certain antidepressant medicines called monoamine oxidase inhibitors (MAOIs) or other medicines that may increase blood pressure,
- if the patient has heart disease (e.g., long QT syndrome), coronary artery disease, or high blood pressure,
- if the patient has a pheochromocytoma (a tumor of the adrenal gland),
- if the patient has metabolic disorders, such as hyperthyroidism or diabetes,
- if the patient has an enlarged prostate,
- if the patient has porphyria (a metabolic disorder that affects the skin and/or nervous system),
- if the patient has increased intraocular pressure, especially narrow-angle glaucoma. Direct contact with the eyes should be avoided.
Prolonged use of a nasal spray containing xylometazoline (longer than 7 days) may lead to chronic swelling, and ultimately to damage to the nasal mucosa (tissue inside the nose).
Children
Xylometazolin Teva 1 mg/ml is not intended for use in children under 10 years of age.
Xylometazolin Teva and other medicines
The patient should tell the doctor or pharmacist about all medicines being taken or recently taken, as well as any medicines planned to be taken.
Concomitant use of Xylometazolin Teva with certain antidepressant medicines (MAOIs of the tranylcypromine type or tricyclic antidepressants), as well as medicines that increase blood pressure, may lead to increased blood pressure.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine.
Since the safety of using Xylometazolin Teva during pregnancy and breastfeeding has not been sufficiently documented, Xylometazolin Teva may only be used after consulting a doctor and carefully weighing the benefits and risks.
Overdose may disrupt fetal blood supply or reduce milk production, and therefore during pregnancy and breastfeeding, the recommended dose should not be exceeded.
Driving and using machines
The medicine should not affect the ability to drive or use machines.
3. How to use Xylometazolin Teva
This medicine should always be used exactly as directed by a doctor or pharmacist. In case of doubt, a doctor or pharmacist should be consulted.
Unless the doctor has prescribed otherwise, the typical dose for adults and children aged 10 and over is 1-2 sprays of Xylometazolin Teva 1 mg/ml into each nostril, as needed, but no more than3 times a day.
Method of administration
After removing the protective cap, place the tip of the applicator in the nostril and press the pump once. During administration, breathe gently through the nose. After use, the dispenser should be wiped clean with a hygienic tissue and the protective cap should be replaced.
Before using the medicine, patients are advised to gently clean their nose. The last daily dose should be administered before bedtime.
For hygiene reasons and to avoid infection, each bottle with the spray should be used by only one person.
Notes:
Before the first administration and after a break in treatment lasting more than 15 days, press the pump several times until a fine mist is released. When using it again, the pump will be ready for immediate use.
Duration of treatment
Xylometazolin Teva should not be used for more than 7 days, unless a doctor has prescribed otherwise.
Before reusing the medicine, a few days should be waited.
In the case of chronic rhinitis, the medicine can only be used under medical supervision, due to the risk of atrophy of the nasal mucosa (damage to the tissue inside the nose).
If the patient thinks that the effect of Xylometazolin Teva is too strong or too weak,
they should consult a doctor or pharmacist.
Using more than the recommended dose of Xylometazolin Teva
In case of significant overdose or accidental use, both in children and adults, a doctor should be consulted immediately. Observation and hospital treatment may be necessary.
The following side effects may occur:
- dilated or constricted pupils
- nausea and vomiting
- pallor, cyanosis of the skin and lips
- fever, sweating, or decreased body temperature
- circulatory problems, such as slow, rapid, or irregular heartbeat, increased or decreased blood pressure
- respiratory arrest (apnea)
- lethargy, drowsiness, coma
- anxiety, agitation, hallucinations, and seizures.
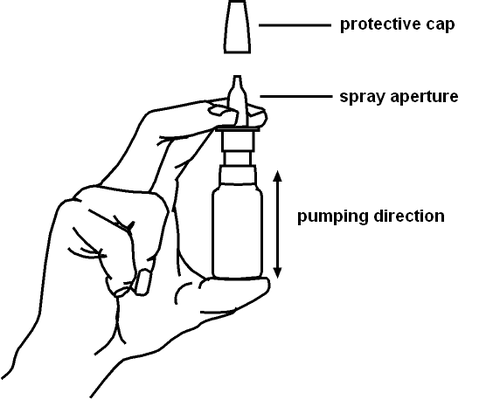
protective cap
applicator
direction of administration
Overdose, especially in children, can often lead to the following symptoms: seizures and coma, slow heart rate, respiratory arrest (apnea), and increased blood pressure, followed by a decrease in blood pressure.
Missing a dose of Xylometazolin Teva
A double dose should not be used to make up for a missed dose. The next dose should be taken according to the dosing schedule described in the package leaflet.
In case of any further doubts about using this medicine, a doctor or pharmacist should be consulted.
4. Possible side effects
Like all medicines, Xylometazolin Teva can cause side effects, although not everybody gets them.
Side effects have been ranked according to the following frequency:Bvery common: may affect more than 1 in 10 people
Common: may affect up to 1 in 10 people
Uncommon: may affect up to 1 in 100 people
Rare: may affect up to 1 in 1,000 people
Very rare: may affect up to 1 in 10,000 people
Not known: frequency cannot be estimated from the available data
Common
- mild, transient symptoms of irritation, such as burning or dryness of the nasal mucosa (tissue inside the nose) and/or throat
- sneezing
Uncommon
allergic reactions (skin rash, itching, swelling of the skin and mucous membranes)
after the effect has stopped, a strong feeling of "stuffiness" of the nose
nasal bleeding
Rare
- rapid heartbeat
- increased blood pressure
- palpitations
- nausea
Very rare
- nervousness (irritability)
- difficulty sleeping or staying asleep (insomnia)
- drowsiness/drowsiness (mainly in children)
- headache
- dizziness
- hallucinations or seizures (mainly in children)
- irregular heartbeat
- respiratory arrest (apnea) in infants and newborns
Reporting side effects
If any side effects occur, including any possible side effects not listed in the package leaflet, the doctor or pharmacist should be informed. Side effects can be reported directly to the Department of Pharmacovigilance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be gathered on the safety of the medicine.
5. How to store Xylometazolin Teva
The medicine should be stored out of sight and reach of children.
This medicine should not be used after the expiry date stated on the carton and label. The expiry date refers to the last day of that month.
The medicinal product does not require special storage conditions.
After the first opening, Xylometazolin Teva should be used within 1 year.
Medicines should not be disposed of via wastewater or household waste. A pharmacist should be asked how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the package and other information
What Xylometazolin Teva contains
- The active substance of the medicine is xylometazoline hydrochloride. Each dose (corresponding to 0.09 ml of solution) contains 0.09 mg of xylometazoline hydrochloride.
- The other ingredients are: citric acid monohydrate, sodium citrate, glycerol 85%, water for injections.
What Xylometazolin Teva looks like and contents of the package
Xylometazolin Teva 1 mg/mlis a clear, almost colorless solution available in a brown glass bottle containing 10 ml (at least 81 doses) of solution with a spray pump, nasal applicator, and protective cap in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Teva Pharmaceuticals Polska Sp. z o.o.
ul. Emilii Plater 53
00-113 Warsaw
Phone: (22) 345 93 00
Manufacturer
Teva Operations Poland Sp. z o.o, ul. Mogilska 80, 31-546 Kraków
Merckle GmbH, Ludwig-Merckle Strasse 3, DE-89143 Blaubeuren, Germany
Pharmachemie B.V., Svensweg 5, NL-2031 GA Haarlem, Netherlands
Teva Czech industries s.r.o., Ostravska 29, c.p. 305, 74770 Opava-Komarov, Czech Republic
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Belgium
Xyloteva 1,0 mg/ml Neusspray, oplossing
Bulgaria
Xylometazolin Teva 1 mg/ml nasal spray, solution
Estonia
Xylometazoline Teva 1,0 mg/ml
Netherlands
Xylometazoline HCl Teva 1,0 mg/ml, neusspray, oplossing
Lithuania
Xylometazoline Teva 1 mg/ml nasal spray nosies purškalas
Latvia
Xylometazoline TEVA 1 mg/ml deguna aerosols, šķīdums
Germany
Nasenspray Erwachsene AbZ 1,0mg/ml Nasenspray Lösung
Poland
Xylometazolin Teva
Slovakia
Lyntol 1,0 mg/ml
Date of last revision of the package leaflet: June 2023
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterMerckle GmbH Pharmachemie B.V. Teva Czech Industries s.r.o. Teva Operations Polska Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Xilometazolin TevaDosage form: Aerosol, 1mg/mlActive substance: xylometazolineManufacturer: Jadran-Galenski laboratorij d.d.Prescription not requiredDosage form: Aerosol, 0.5 mg/mlActive substance: xylometazolineManufacturer: Basic Pharma Manufacturing B.V.Prescription not requiredDosage form: Aerosol, 1 mg/mlActive substance: xylometazolineManufacturer: Basic Pharma Manufacturing B.V.Prescription not required
Alternatives to Xilometazolin Teva in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Xilometazolin Teva in Ukraine
Alternative to Xilometazolin Teva in Spain
Online doctors for Xilometazolin Teva
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Xilometazolin Teva – subject to medical assessment and local rules.








