
Xedine Ha

Ask a doctor about a prescription for Xedine Ha

How to use Xedine Ha
Package Leaflet: Information for the Patient
Xedine HA, 1 mg/ml, Nasal Spray, Solution
Xylometazoline Hydrochloride
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this package leaflet for the patient, or as directed by a doctor or pharmacist.
- This package leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any not listed in this package leaflet, the doctor or pharmacist should be informed. See section 4.
- If there is no improvement after 3 days or the patient feels worse, a doctor should be consulted.
Table of Contents of the Package Leaflet
- 1. What is Xedine HA and what is it used for
- 2. Important information before using Xedine HA
- 3. How to use Xedine HA
- 4. Possible side effects
- 5. How to store Xedine HA
- 6. Package contents and other information
1. What is Xedine HA and what is it used for
Xedine HA belongs to a group of sympathomimetic medicines used in nasal diseases. This medicine contains xylometazoline hydrochloride, which helps to narrow the blood vessels in the nose, thereby reducing the swelling of the nasal mucosa. Xedine HA also contains hyaluronic acid (in the form of sodium hyaluronate), which protects and moisturizes the nasal mucosa. Xedine HA is used to reduce the swelling of the nasal mucosa caused by inflammation of the nasal mucosa (rhinitis) or inflammation of the paranasal sinuses (sinusitis). Xedine HA at a strength of 1 mg/ml is indicated for use in adults and children from 12 years of age.
2. Important information before using Xedine HA
When not to use Xedine HA:
if the patient is allergic to xylometazoline hydrochloride or any of the other ingredients of this medicine (listed in section 6); if the patient has increased intraocular pressure, especially in the case of narrow-angle glaucoma; if the patient has chronic rhinitis (long-term nasal irritation) with little or no discharge; if the patient is taking or has taken monoamine oxidase inhibitors (MAOIs) in the last two weeks; if the patient is taking other medicines that may increase blood pressure; if the patient has inflammation caused by hypersensitivity of the nasal blood vessels; if the patient has an inflammatory condition associated with thinning of the nasal membranes with or without discharge; if the patient has undergone brain surgery through the nose or mouth. Xedine HA is not intended for children under 12 years of age.
Warnings and precautions
Beforestarting to use this medicine, the patient should consult a doctor if they have a strong reaction to sympathomimetics (substances similar to adrenaline), as the use of Xedine HA in such patients may cause insomnia, dizziness, uncontrolled muscle tremors, irregular heartbeat, or increased blood pressure; heart disease (e.g., long QT syndrome), vascular disease, or high blood pressure; hyperthyroidism, diabetes, or other metabolic disorders; adrenal disease; or prostate enlargement (prostate hypertrophy). Continuous use of Xedine HA for a long periodmay lead to worsening of nasal congestion symptoms.
Xedine HA and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. This medicine should not be taken while using certain antidepressants, such as tricyclic and tetracyclic antidepressants, monoamine oxidase inhibitors, and within two weeks of using monoamine oxidase inhibitors (see "When not to use Xedine HA"); or other medicines that may increase blood pressure.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine. Xedine HA should not be used during pregnancy due to the lack of data on the use of xylometazoline hydrochloride in pregnant women. As there are no data on the penetration of xylometazoline hydrochloride into human milk, this medicine should not be used during breastfeeding.
Driving and using machines
The effect of Xedine HA on the ability to drive and use machines is not known.
3. How to use Xedine HA
This medicine should always be used exactly as described in the package leaflet for the patient, or as directed by a doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
Adults and children from 12 years of age:
Unless otherwise directed by a doctor or pharmacist, one dose of Xedine HA should be used in each nostril, up to 3 times a day, for no longer than 7 days.
| Before using the medicine, the patient should clean their nose. The protective cap should be removed, as shown in Figure 1. | Figure 1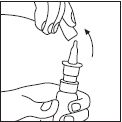 |
| The bottle should be held between the index and middle fingers, with the thumb supporting the base of the bottle, as shown in Figure 2. To spray the dose, the pump should be pressed towards the base of the bottle. Before the first use, the aerosol should be sprayed into the air several times (five times) to obtain a uniform dose. If the medicine has not been used for several days, at least one dose of the aerosol should be sprayed into the air to obtain a uniform dose. | Figure 2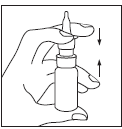 |
| The upper part of the dosing pump should be inserted into the nostril, as shown in Figure 3 (the aerosol should not be sprayed under the nostril). By pressing the pump once, the patient should inhale through the nose while gently closing the other nostril with the index finger of the other hand. The pump should be released and removed from the nostril. This process should be repeated in the other nostril. After use, the dosing pump should be cleaned with a dry and clean sanitary wipe and the protective cap should be put back on. | Figure 3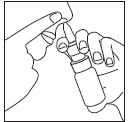 |
To minimize the risk of spreading infections, the medicine packaging should not be used by more than one person, and the applicator should be rinsed after each use. This medicine should not be used in children under 12 years of age.
Using more than the recommended dose of Xedine HA
A doctor, pharmacist, or the nearest emergency department should be contacted immediately, taking the medicine packaging or this package leaflet with them. The following symptoms of overdose may occur (mainly in children):
- severe central nervous system depression
- drowsiness
- dry mouth
- sweating
- rapid heartbeat
- irregular heartbeat
- increased blood pressure.
Missing a dose of Xedine HA
If a dose of the medicine is missed, it should be taken as soon as the patient remembers. If it is almost time for the next dose, the missed dose should be skipped. The next dose should be taken at the usual time. A double dose should not be taken to make up for a missed dose. If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The use of Xedine HA should be stopped and a doctor consulted immediatelyif any of the following side effects occur, which may be symptoms of an allergic reaction:
- difficulty breathing or swallowing, swelling of the face, lips, tongue, or throat
- severe skin itching with red rash or bulging blisters.
Other side effects:
Frequent(may occur in less than 1 in 10 people)
feeling of tingling or burning in the nose and throat, as well as dryness of the nasal mucosa
Rare(may occur in less than 1 in 1,000 people)
headache, high blood pressure, nervousness, nausea, dizziness, insomnia, and palpitations
transient vision disturbances and systemic allergic reactions
Frequency not known(frequency cannot be estimated from the available data):
- worsening of nasal congestion symptoms after stopping the use of the medicine
- nasal bleeding.
Reporting side effects
If any side effects occur, including any not listed in the package leaflet, the doctor or pharmacist should be informed. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49 21 301, fax: 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the use of this medicine.
5. How to store Xedine HA
The medicine should be stored out of sight and reach of children. There are no special precautions for storage. The shelf life after opening the bottle is 6 months. This medicine should not be used after the expiry date stated on the carton and bottle after the abbreviation: EXP. The expiry date refers to the last day of the specified month. The batch number is stated after the abbreviation: Lot. Medicines should not be disposed of via wastewater or household waste. A pharmacist should be asked how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What Xedine HA contains
- The active substance of the medicine is xylometazoline hydrochloride. Each 1 ml of Xedine HA contains 1 mg of xylometazoline hydrochloride. Each dose (140 microliters) contains 0.140 mg of xylometazoline hydrochloride.
- The other ingredients (excipients) are: purified sea water, potassium dihydrogen phosphate, sodium hyaluronate, purified water.
What Xedine HA looks like and what the package contains
Clear, colorless, sterile solution. Does not contain preservatives. White plastic bottle containing 10 ml of solution, closed with a dosing pump and nasal applicator, and a protective cap, placed in a cardboard box.
Marketing authorization holder:
Ranbaxy (Poland) Sp. z o.o. ul. Idzikowskiego 16, 00-710 Warsaw
Manufacturer:
Jadran-Galenski laboratorij d.d. Svilno 20, 51000 Rijeka, Croatia; S.C. Terapia SA, 124 Fabricii Street, 400 632 Cluj Napoca, Romania
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Latvia: Xylometazolini hydrochloridum Ranbaxy; Romania: RHINXYL HA; Date of last revision of the package leaflet: 31.05.2023
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterJadran-Galenski laboratorij d.d. S.C. Terapia S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Xedine HaDosage form: Aerosol, 1mg/mlActive substance: xylometazolineManufacturer: Jadran-Galenski laboratorij d.d.Prescription not requiredDosage form: Aerosol, 0.5 mg/mlActive substance: xylometazolineManufacturer: Basic Pharma Manufacturing B.V.Prescription not requiredDosage form: Aerosol, 1 mg/mlActive substance: xylometazolineManufacturer: Basic Pharma Manufacturing B.V.Prescription not required
Alternatives to Xedine Ha in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Xedine Ha in Ukraine
Alternative to Xedine Ha in Spain
Online doctors for Xedine Ha
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Xedine Ha – subject to medical assessment and local rules.








