
Vizitrav

Ask a doctor about a prescription for Vizitrav

How to use Vizitrav
Package Leaflet: Information for the User
Vizitrav, 40 micrograms/ml, eye drops, solution
Trawoprost
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- -Keep this package leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including those not listed in this package leaflet, they should inform their doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet
- 1. What is Vizitrav and what is it used for
- 2. Important information before using Vizitrav
- 3. How to use Vizitrav
- 4. Possible side effects
- 5. How to store Vizitrav
- 6. Contents of the pack and other information
1. What is Vizitrav and what is it used for
Vizitrav contains trawoprost, a medicine belonging to a group called prostaglandin analogues. It works by
reducing the pressure in the eye. Vizitrav can be used alone or in combination with other
eye drops, such as beta-blockers, which also reduce the pressure inside the eye.
Vizitrav is used to reduce high pressure in the eye in adults, adolescents, and children from 2 months of age. This pressure can lead to the development of a disease called glaucoma.
Vizitrav eye drops, solution is a sterile solution that does not contain preservatives.
2. Important information before using Vizitrav
When not to use Vizitrav
- If the patient is allergic to trawoprost or any of the other ingredients of this medicine (listed in section 6).
In such cases, consult a doctor for advice.
Warnings and precautions
Before starting to use Vizitrav, discuss it with a doctor or pharmacist.
- Vizitrav may increasethe length, thickness, color, and/or number of eyelashes. Changes in the eyelids, including excessive hair growth and changes in the tissues around the eye, have also been observed.
- Vizitrav may change the color of the iris(the colored part of the eye). The change may be permanent. It is also possible to change the color of the skin around the eye.
- If the patient has had cataract surgery, they should consult a doctor before starting to use Vizitrav.
and
If the patient currently has or has had eye inflammation(inflammation of the iris and/or inflammation of the vascular membrane of the eye), they should consult a doctor before starting to use Vizitrav.
- Vizitrav may rarely cause wheezing or shortness of breathor exacerbate asthmasymptoms. If changes in breathing are observed while using Vizitrav, the patient should immediately inform their doctor.
- Trawoprost may be absorbed through the skin. If any amount of the medicine comes into contact with the skin, it should be immediately rinsed with water. This is especially important for pregnant or breastfeeding women.
- If the patient uses soft contact lenses, they should not use the medicine with the lenses in place. After administering the medicine, they should wait 15 minutes before reinserting the lenses.
Children and adolescents
Vizitrav can be used in children from 2 months to less than 18 years of age in the same doses as for adults. Using Vizitrav in children under 2 months of age is not recommended.
Vizitrav and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Pregnancy, breastfeeding, and fertility
Pregnant women should not use Vizitrav. If the patient suspects they may be pregnant, they should inform their doctor. Women who may become pregnant during treatment with this medicine must use appropriate contraceptive methods.
Vizitrav should not be used during breastfeeding. Vizitrav may pass into breast milk.
Before using any medicine, the patient should consult a doctor.
Driving and using machines
After administering Vizitrav, vision may be blurred for a while. The patient should not drive or operate machines until these symptoms have resolved.
Vizitrav contains macrogolglycerol hydroxystearate 40
This medicine contains macrogolglycerol hydroxystearate 40, which may cause skin reactions.
3. How to use Vizitrav
This medicine should always be used as directed by a doctor, the doctor treating the child, or a pharmacist. In case of doubts, the patient should consult a doctor, the doctor treating the child, or a pharmacist.
The recommended dose isone drop into the affected eye or eyes, once daily in the evening.
Vizitrav can be used in both eyes only after receiving such a recommendation from a doctor.
The medicine should be used for as long as the doctor or the doctor treating the child recommends.
Vizitrav should only be used for eye drops for the patient or the child.
Method of administration
1a 1b |
|
2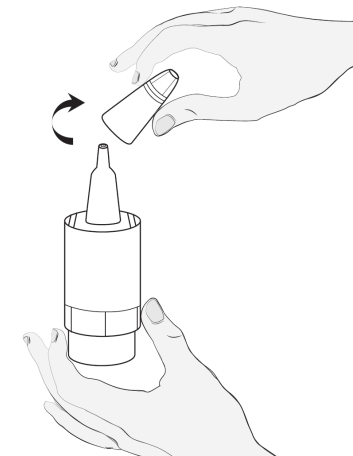 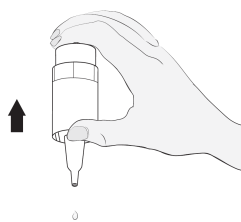 |
|
3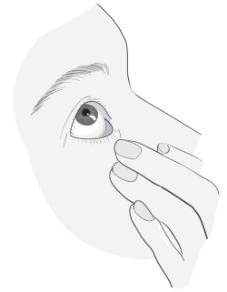 |
|
4 5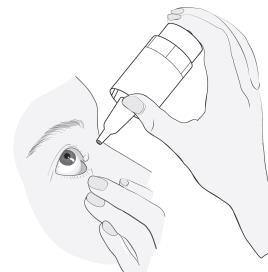 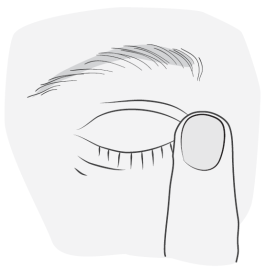 |
|
|
If the patient is using other eye medicines, such as eye drops or ointment,
they should wait at least 5 minutes between administering Vizitrav and using other eye medicines.
Using more Vizitrav than recommended
The patient should flushthe entire amount of the medicine from the eye with warm water. They should not administer the next drop until it is time for the next dose.
Missing a dose of Vizitrav
The patient should use the next dose at the planned time. They should not use a double dose
to make up for a missed dose. They should never use more than one drop into the affected eye (or eyes) once daily.
Stopping the use of Vizitrav
The patient should not stop using Vizitrav without first consulting their doctor or the doctor treating their child, as the pressure inside the patient's eye may not be controlled, which can lead to vision loss.
If the patient has any further doubts about using this medicine, they should consult a doctor, the doctor treating their child, or a pharmacist.
4. Possible side effects
Like all medicines, Vizitrav can cause side effects, although not everybody gets them.
In general, it is possible to continue using the drops, as long as the side effects are not severe. If the patient is concerned, they should contact their doctor or pharmacist. Without consulting a doctor, the patient should not stop using Vizitrav.
The following side effects have been observed during the use of trawoprost.
Very common side effects (may affect more than 1 in 10 people)
Eye disorders:
- eye redness.
Common side effects (may affect up to 1 in 10 people)
Eye disorders:
- change in iris color (the colored part of the eye)
- eye pain
- eye discomfort
- eye dryness
- eye itching
- eye irritation.
Uncommon side effects (may affect up to 1 in 100 people)
Eye disorders:
- corneal disorders
- eye inflammation
- uveitis
- inflammation inside the eye
- inflammation of the eye surface with or without damage to the eye surface
- photophobia
- eye discharge
- blepharitis
- eyelid redness
- periorbital edema
- eyelid itching
- blurred vision
- increased tear production
- conjunctivitis
- ectropion
- eye clouding
- eyelid margin crusting
- excessive eyelash growth
General disorders:
- exacerbated allergic reactions
- headache
- irregular heartbeat
- cough
- nasal congestion
- throat irritation
- periorbital skin darkening
- skin darkening
- abnormal hair growth
- hypertrichosis
Rare side effects (may affect up to 1 in 1,000 people)
Eye disorders:
- flashing lights
- eyelid rash
- abnormal eyelash positioning, with eyelashes growing in towards the eye
- eye edema
- reduced visual acuity
- halo vision
- decreased corneal sensitivity
- meibomian gland inflammation
- intraocular pigmentation
- pupil dilation
- eyelash thickening
- eyelash color change
- eye fatigue General disorders:
- ocular viral infection
- dizziness
- unpleasant taste
- irregular or slow heartbeat
- increased or decreased blood pressure
- shortness of breath
- asthma
- allergic rhinitis
- nasal dryness
- voice changes
- gastrointestinal discomfort or ulcer
- constipation
- dry mouth
- skin redness or itching
- rash
- hair color changes
- eyelash loss
- joint pain
- musculoskeletal pain
- general weakness.
Frequency not known (frequency cannot be estimated from the available data)
Eye disorders:
- posterior segment inflammation
- enophthalmos.
General disorders:
- depression
- anxiety
- insomnia
- abnormal sensation
- tinnitus
- chest pain
- irregular heartbeat
- accelerated heartbeat
- asthma exacerbation
- diarrhea
- epistaxis
- abdominal pain
- nausea
- vomiting
- pruritus
- abnormal hair growth
- painful or involuntary urination
- increased prostate-specific antigen.
Additional side effects in children and adolescents
The most common side effects observed in children and adolescents using trawoprost were eye redness and excessive eyelash growth. Both of these side effects were observed more frequently in children and adolescents compared to adults.
Reporting side effects
If the patient experiences any side effects, including those not listed in the package leaflet, they should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Tel.: +48 22 49-21-301
Fax: +48 22 49-21-309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, it is possible to gather more information on the safety of the medicine.
5. How to store Vizitrav
The medicine should be stored out of sight and reach of children.
Do not use Vizitrav after the expiry date stated on the label and carton after "Exp". The expiry date refers to the last day of the month.
Do not use the medicine if, before the first opening, it is noticed that the bottle is damaged or broken.
Before opening: Store in a temperature below 25°C.
There are no special precautions for storage of the medicine after the first opening.
The bottle should be discarded 28 days after it is first openedto prevent infections and a new bottle should be used. The opening date of the bottle should be written in the space provided on the label of each bottle and on the carton.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Vizitrav contains
- The active substance is trawoprost 40 micrograms/ml.
- The other ingredients are: macrogolglycerol hydroxystearate 40, boric acid, mannitol (E421), sodium chloride, propylene glycol, sodium hydroxide, and purified water.
What Vizitrav looks like and contents of the pack
A multidose container made of PP with a pump (made of PP, HDPE, LDPE) and a cap made of HDPE, placed in a pressure cylinder. The whole thing is in a carton.
Vizitrav eye drops, solution is available in the following pack sizes:
1 x 2.5 ml (a single multidose container containing 2.5 ml of solution)
3 x 2.5 ml (three multidose containers containing 2.5 ml of solution)
4 x 2.5 ml (four multidose containers containing 2.5 ml of solution).
Cartons containing 1 or 3 bottles.
Not all pack sizes may be marketed.
Marketing authorization holder:
Bausch + Lomb Ireland Limited
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
Manufacturer:
JADRAN - GALENSKI LABORATORIJ d.d.
Svilno 20
51000 Rijeka
Croatia
PHARMATHEN S.A.
Dervenakion 6
15351 Pallini Attikis
Greece
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria
Vizitrav 40 Mikrogramm/ml Augentropfen, Lösung
Belgium
Vizitrav 40 microgram/ml oogdruppels, oplossing
Bulgaria
Vizitrav 40 μg/ml капки за очи, разтвор
Cyprus
Vizitrav Οφθαλμικές σταγόνες διάλυμα 40 µg/ml
Croatia
Vizitrav 40 mikrograma/ml, kapi za oko, otopina
Denmark
Vizitrav 40 micrograms/ml, eye drops, solution (øjendråber, opløsning)
Estonia
Vizitrav 40 mikrogrammi/ml silmatilgad, lahus
France
Vizitrav, 40 microgrammes/ml, solution eye drops
Greece
Vizitrav 40 μικρογραμμάρια/ml οφθαλμικές σταγόνες, διάλυμα
Spain
Vizitrav 40 μg/ml, colirio en solución
Netherlands
Vizitrav 40 microgram/ml oogdruppels, oplossing
Lithuania
Vizitrav 40 mikrogramų/ml akių lašai (tirpalas)
Luxembourg
Vizitrav 40 microgrammes/ml collyre en solution
Germany
Vizitrav 40 Mikrogramm/ml Augentropfen, Lösung
Poland
Vizitrav 40 mikrogramów/ml, krople do oczu, roztwór
Portugal
Vizitrav 0.04 mg/ml colírio, solução
Date of last revision of the package leaflet:November 2024
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterJadran-Galenski laboratorij d.d. Pharmathen S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VizitravDosage form: Drops, 40 mcg/mlActive substance: travoprostPrescription requiredDosage form: Drops, 40 mcg/mlActive substance: travoprostManufacturer: Jadran-Galenski laboratorij d.d.Prescription requiredDosage form: Drops, 40 mcg/mlActive substance: travoprostPrescription required
Alternatives to Vizitrav in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Vizitrav in Hiszpania
Alternative to Vizitrav in Ukraina
Online doctors for Vizitrav
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Vizitrav – subject to medical assessment and local rules.














