
Vidisic

Ask a doctor about a prescription for Vidisic

How to use Vidisic
Leaflet attached to the packaging: patient information
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Vidisic
2 mg/g, eye gel
Carbomer
It is essential to carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist.
- The leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
- If there is no improvement or the patient feels worse, they should contact their doctor.
Table of contents of the leaflet
- 1. What is Vidisic and what is it used for
- 2. Important information before using Vidisic
- 3. How to use Vidisic
- 4. Possible side effects
- 5. How to store Vidisic
- 6. Contents of the packaging and other information
1. What is Vidisic and what is it used for
The active substance of Vidisic is carbomer, a high molecular weight water-binding compound. The medicine is characterized by high viscosity and physiological pH, close to that of natural tears.
It replaces natural tears in cases of disturbed secretion. Vidisic is used for the symptomatic treatment of dry eye syndrome (also known as dry keratoconjunctivitis).
Properties of Vidisic
Vidisic is a liquid gel that binds water, forming a transparent protective layer on the surface of the eye, providing adequate moisturizing of the cornea and conjunctiva. It is easy to use if used according to the administration instructions.
What is dry eye syndrome?
Dry eye syndrome is an eye condition in which the surface of the eye is not properly moisturized. This can be caused by reduced production of natural tears, their abnormal composition, or excessive evaporation. If the amount or composition of the tear film is disturbed, the cornea and conjunctiva become dry, resulting in burning, feeling of dryness, feeling of sand in the eye, feeling of pressure, and hypersensitivity to light.
Dry eye syndrome is a very common eye condition. It can be caused by many factors, including working in an air-conditioned room, working on a computer, environmental pollution, taking certain medications, hormonal changes during menopause, or decreased tear production in older age.
Page 1 of 5
2. Important information before using Vidisic
When not to use Vidisic
- if the patient is allergic to the active substance or to any of the excipients (listed in section 6).
Warnings and precautions
Before starting to use Vidisic, the patient should discuss it with their doctor or pharmacist.
If the symptoms of dry eye syndrome persist or worsen, the patient should stop using the medicine and consult their doctor.
If the patient wears contact lenses, they should remove them before using Vidisic.
Contact lenses can be put back in 15 minutes after administering Vidisic.
Vidisic is a sterile medicine until the first opening. It is very important to keep the tube clean and not to contaminate its contents. During use, the patient should pay special attention to avoid touching the eyes, eyelids, and other surfaces with the tube tip (see also section 3: "Administration instructions").
Children and adolescents
There are no available data. No clinical trials have been conducted on the use of Vidisic in children and adolescents.
Important information about some ingredients of Vidisic
Vidisic contains cetrimide as a preservative, which can cause eye irritation (burning, redness, feeling of a foreign body) and may damage the corneal epithelium if used frequently or for a long period. If the above side effects occur, the patient should stop using Vidisic and consult their doctor or pharmacist, who will recommend using other medicines without preservatives.
Vidisic and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
No studies have been conducted to determine whether Vidisic affects the action of other medicines or whether other medicines may affect the action of Vidisic.
Warning:
Vidisic may prolong the contact time of other eye medicines with the surface of the eye.
If it is necessary to administer another eye medicine or medicines at the same time as Vidisic, the patient should wait at least 5 minutes between administering the medicines. If Vidisic is used in addition to eye ointment, the interval between administering the medicines should be 15 minutes. Vidisic should always be used last.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Pregnancy
Due to the lack of data on the use of carbomer in pregnant women, it is recommended to avoid using Vidisic during pregnancy.
Page 2 of 5
Breastfeeding
It is not known whether carbomer or its metabolites pass into breast milk. Therefore, it is not recommended to use Vidisic during breastfeeding, unless the doctor decides otherwise.
Fertility
There are no data on the effect on fertility.
Driving and using machines
Vidisic has a moderate effect on the ability to drive and use machines.
Immediately after administration, the medicine may cause blurred vision for a short period, resulting in unclear vision. The patient should not drive, operate machinery, or perform potentially hazardous activities until their vision is clear.
3. How to use Vidisic
This medicine should always be used exactly as described in the patient leaflet or as directed by a doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
Recommended dose
The dosage for the treatment of dry eye syndrome depends on the individual patient's condition.
Drops should be administered into the conjunctival sac 3 to 5 times a day or as needed, and about 30 minutes before sleep (otherwise, there is a risk of eyelid adhesion).
The patient should consult an ophthalmologist during the use of Vidisic for the treatment of dry eye syndrome, which usually requires long-term or continuous treatment.
If the patient feels that the effect of Vidisic is too strong or too weak, they should consult their doctor or pharmacist.
Administration instructions:
Warning!
The patient should not touch the tube tip with their fingers or touch the tube tip to the surface of the eye or any other surface, as this may contaminate the contents of the tube. Using contaminated eye medicines can lead to serious vision damage, including vision loss.
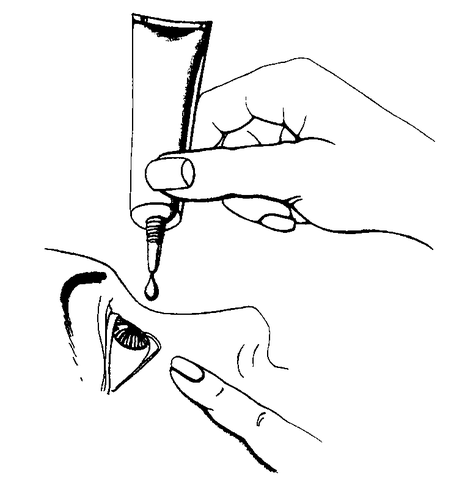
- 1. Wash hands thoroughly.
- 2. Remove the protective cap.
- 3. Tilt the head back.
- 4. Hold the tube vertically over the eye, holding it with the thumb and index finger.
- 5. With the index finger of the other hand, gently pull down the lower eyelid to create a "pocket" between the eyeball and the eyelid, into which the gel will be administered.
- 6. Bring the tube tip close to the eye, without touching the tube tip to the eye, eyelid, or other surfaces.
- 7. Gently press the tube to release 1 drop.
- 8. Looking up, administer 1 drop of gel into the created "pocket". If the drop does not get into the eye, repeat the action.
- 9. Several times, gently close and open the eye to spread the gel over the entire surface.
- 10. If the gel is to be administered to both eyes, repeat the above steps for the second eye.
- 11. Immediately after use, carefully close the tube.
Having someone else help or using a mirror may make it easier to administer the medicine.
Page 3 of 5
Vidisic is available in a tube with a flat cap, allowing the tube to be stored in a vertical position.
Using a higher dose of Vidisic than recommended
If a higher dose of Vidisic is accidentally used than recommended, it may cause temporary vision disturbances, which will quickly resolve.
Missing a dose of Vidisic
If a dose is missed, the next dose should be taken at the usual time, according to the recommended dosing schedule. A double dose should not be used to make up for a missed dose.
In case of any further doubts about using this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Vidisic can cause side effects, although not everybody gets them.
Very rare side effects (may affect up to 1 in 10,000 people):
- eye burning
- eye redness
- eyelid rash
- feeling of a foreign body in the eye
- giant papillary conjunctivitis
- eye itching
- feeling of stickiness in the eye
- superficial punctate keratitis
- tearing
- blurred vision.
The above reactions, including allergic reactions, may be caused by the preservative contained in the medicine (cetrimide) or may be related to intolerance to one of the other ingredients of the medicine. Cetrimide may also cause damage to the corneal epithelium.
Blurred vision after administering Vidisic may be related to the high viscosity of the medicine.
Children and adolescents
There are no data.
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, the patient should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: +48 (22) 49 21 301
fax: +48 (22) 49 21 309
website: https://smz.ezdrowie.gov.pl
Reporting side effects will help to gather more information on the safety of the medicine.
Page 4 of 5
5. How to store Vidisic
The medicine should be stored out of sight and reach of children.
There are no special precautions for storage.
The medicine should not be used after the expiry date stated on the outer or immediate packaging. The expiry date is the last day of the stated month.
Unused medicine should be discarded 6 weeks after the first opening of the tube.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Vidisic contains
The active substance of Vidisic is carbomer. 1 g of gel contains 2 mg of carbomer.
The other ingredients of the medicine are: cetrimide, sodium hydroxide, sorbitol, water for injections.
What Vidisic looks like and what the packaging contains
Vidisic is an eye gel.
The medicine is available in a tube containing 10 g of gel in a cardboard box.
Shelf life after first opening the tube: 6 weeks
For more detailed information, the patient should contact the marketing authorization holder or parallel importer:
Marketing authorization holder in Germany, the country of export:
Dr. Gerhard Mann
Chem.-pharm. Fabrik GmbH
Brunsbütteler Damm 165-173
13581 Berlin, Germany
Manufacturer:
Dr. Gerhard Mann
Chem.-pharm. Fabrik GmbH
Brunsbütteler Damm 165-173
13581 Berlin, Germany
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
German export license number: 40612.00.00
Parallel import license number: 607/12
Date of leaflet approval:05.10.2022
[Information about the trademark]
Page 5 of 5
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Dr. Gerhard Mann
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VidisicDosage form: Drops, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsPrescription not requiredDosage form: Gel, 2.5 mg/gActive substance: artificial tears and other indifferent preparationsManufacturer: Santen OyPrescription not requiredDosage form: Drops, 50 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Ursapharm Arzneimittel GmbHPrescription required
Alternatives to Vidisic in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Vidisic in Ukraine
Alternative to Vidisic in Spain
Online doctors for Vidisic
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Vidisic – subject to medical assessment and local rules.














