
Ventolin Disk

Ask a doctor about a prescription for Ventolin Disk

How to use Ventolin Disk
Leaflet attached to the packaging: patient information
Ventolin Disk, 200 mcg/inhalation dose, inhalation powder
Salbutamol
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others.
- The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Ventolin Disk and what is it used for
- 2. Important information before using Ventolin Disk
- 3. How to use Ventolin Disk
- 4. Possible side effects
- 5. How to store Ventolin Disk
- 6. Contents of the packaging and other information
1. What is Ventolin Disk and what is it used for
Ventolin Disk, inhalation powder, contains the active substance salbutamol. It belongs to a group of fast-acting bronchodilators.
Bronchodilators:
- help maintain airway patency, making it easier to breathe.
- help reduce chest tightness, wheezing, and cough associated with asthma. Ventolin Disk, inhalation powder, is used to treat breathing disorders in adults, adolescents, and children aged 4 and over, with asthma and similar conditions, as well as to prevent asthma symptoms caused by physical exertion and contact with allergens (asthma triggers). These include house dust, pollen, cat and dog dander, and tobacco smoke.
2. Important information before using Ventolin Disk
When not to use Ventolin Disk
- if the patient is allergic to salbutamol sulfate or any of the other ingredients of this medicine (listed in section 6),
- in case of threatened abortion,
- to prevent premature labor,
- if the patient has a severe milk protein allergy.
Warnings and precautions
Before starting to use Ventolin Disk, the patient should consult their doctor if they:
- have poorly controlled asthma (e.g., frequent symptoms or exacerbations, or reduced physical fitness). The doctor may recommend starting or increasing the dose of a medicine, such as an inhaled corticosteroid, to control the disease.
- have high blood pressure,
- have hyperthyroidism,
- have had heart problems in the past, such as irregular or rapid heartbeat or chest pain,
- have low potassium levels in the blood,
- are taking xanthine derivatives (e.g., theophylline) or steroids to treat asthma,
- are taking diuretics, sometimes used to treat high blood pressure or heart disease.
If the patient is taking any of these medicines, the doctor will recommend monitoring potassium levels in the blood.
The patient should consult their doctor if any of the above circumstances apply to them.
The patient should consult their doctor if the duration of action of the medicine is less than 3 hours.
Ventolin Disk and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including those available without a prescription.
Some medicines may affect the action of Ventolin Disk or increase the risk of side effects. These include:
- beta-adrenergic receptor blockers, such as propranolol, used to treat high blood pressure or other heart conditions.
The doctor will decide whether the patient can use Ventolin Disk with these medicines.
Pregnancy and breastfeeding
Ventolin Disk is not usually recommended for use during pregnancy.
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine. The doctor will assess whether the patient can take Ventolin Disk during this period.
Driving and using machines
No effect on the ability to drive and use machines has been observed.
Ventolin Disk contains lactose monohydrate
Each dose of Ventolin Disk contains approximately 12.5 mg of lactose monohydrate (which contains milk proteins). In patients with lactose intolerance, this amount of lactose does not usually cause any problems. If the patient has previously been diagnosed with intolerance to some sugars, they should contact their doctor before taking the medicine.
3. How to use Ventolin Disk
Ventolin Disk should always be used as directed by the doctor. If in doubt, the patient should consult their doctor.
Ventolin Disk is intended for inhalation use only through the mouth.
Before use, the patient should read the instructions for using the Disk inhaler device.
Increasing the dose or frequency of use of the medicine is only allowed under medical supervision.
Ventolin Disk should be used as needed, not regularly.
If the patient's asthma is active (e.g., frequent symptoms or exacerbations, such as shortness of breath that interferes with speaking, eating, or sleeping, coughing, wheezing, chest tightness, or reduced physical fitness), they should immediately tell their doctor, who may start treatment or increase the dose of a medicine that helps control asthma symptoms, such as an inhaled corticosteroid.
If the patient thinks the medicine is not working as well as usual, they should tell their doctor as soon as possible (e.g., the patient needs larger doses to relieve breathing problems or asthma symptoms do not improve for at least 3 hours after using the inhaler), because asthma may be worsening and another medicine may be needed.
If the patient uses Ventolin Disk more often than twice a week to relieve asthma symptoms, not including preventive use before physical exertion, it means that asthma is not well controlled and may increase the risk of severe asthma attacks (asthma exacerbations), which can cause serious complications and be life-threatening. The patient should contact their doctor as soon as possible to verify their asthma treatment. If the patient uses a medicine with anti-inflammatory effects in the lungs, such as an "inhaled corticosteroid", it is essential to continue using it regularly, even if the patient feels better.
Adults, adolescents, and children aged 4 and over
- To relieve sudden bronchospasm, one inhalation (200 micrograms of salbutamol) is used.
- For preventive use, the usual dose is one inhalation (200 micrograms of salbutamol) 10-15 minutes before exertion or expected contact with an allergen.
The maximum dose is 4 inhalations (800 micrograms) per day. The patient should not use more inhalations of the medicine or use it more frequently than 4 times a day. The patient should tell their doctor if the medicine does not work as well as before, as this may be due to worsening asthma control and a change in medicine may be necessary.
Instructions for using the Disk inhaler device
The doctor, nurse, or pharmacist should instruct the patient on how to use the Disk inhaler device properly. They should periodically check that the patient is using the Disk inhaler device correctly.
After removing the Disk inhaler device from the packaging for the first time, it is in the closed position.
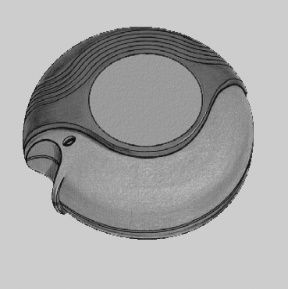
The Disk inhaler device contains Ventolin in the form of consecutive doses of inhalation powder.
The Disk inhaler device has a dose counter that shows how many doses of medicine are left in the inhaler device. The counter shows the dose number down to 0. The numbers from 5 to 0 appear in red to warn that only a few doses of medicine are left in the inhaler device.
If the counter shows 0, it means that the inhaler device is empty.
Using the Disk inhaler device
The patient should follow these steps:
- 1. OPENING: To open the Disk inhaler device, the patient should hold the cover with one hand and place their thumb in the indentation on the case with the other hand. The thumb should be slid along the indentation away from them –
until it clicks. After doing this, a small opening is visible in the mouthpiece.
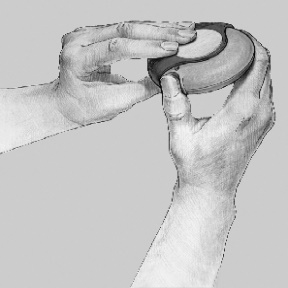
- 2. SETTING THE DOSE: The Disk inhaler device should be set with the mouthpiece facing the patient. It can be held in either the right or left hand. The slide should be pushed away from the patient until it clicks. The Disk inhaler device is now ready for use. Each time the lever is pushed, the next dose of powder is prepared for inhalation. The patient should not play with the slide, as this will open the next dose and reduce the number of doses of medicine available for inhalation.
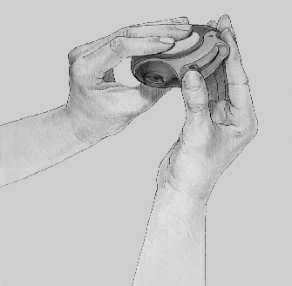
- 3. INHALING: Before inhaling the medicine, the patient should carefully read the following instructions.
- The Disk inhaler device should be held away from the mouth and a slow, deep exhalation should be performed. The patient should not exhale into the Disk inhaler device.
- The mouthpiece should be placed in the mouth; a deep inhalation should be performed from the Disk inhaler device through the mouth, not through the nose.
- The breath should be held for about 10 seconds or as long as is comfortable, then a slow exhalation should be performed.
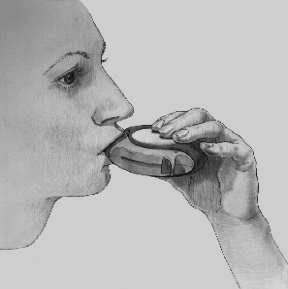
- 4. CLOSING:
- To close the Disk inhaler device, the patient should place their thumb in the indentation on the case and slide it towards them.
- When closing the Disk inhaler device, a "click" is heard. The dose-setting slide automatically returns to its original position. The Disk inhaler device is now ready for reuse.
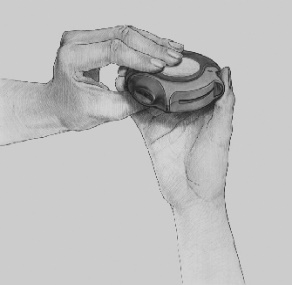
Cleaning
To clean the mouthpiece of the Disk inhaler device, it should be wiped with a dry, soft cloth.
Using a higher than recommended dose of Ventolin Disk
In case of using a higher dose of Ventolin than recommended, the patient should contact their doctor or pharmacist as soon as possible for advice. The patient should have the carton of the medicine with them to show the doctor.
The following symptoms may occur:
- faster than usual heartbeat,
- tremors,
- excessive restlessness. These symptoms usually resolve within a few hours. Additionally, the following may occur:
- decreased potassium levels in the blood (hypokalemia)
- abdominal pain, increased lung ventilation (rapid breathing), shortness of breath despite decreased wheezing, cold hands and feet, irregular heartbeat, thirst (symptoms suggesting the occurrence of lactic acidosis).
Missing a dose of Ventolin Disk
In case of missing a dose of Ventolin Disk, inhalation powder, the patient should take it as soon as possible and then continue taking the medicine as directed by the doctor.
The patient should not take a double dose of the medicine to make up for the missed dose.
If the patient has any further doubts about using the medicine, they should consult their doctor or pharmacist.
4. Possible side effects
If breathing worsens or wheezing increases immediately after taking this medicine, the patient should stop using it and contact their doctor immediately.
Like all medicines, Ventolin can cause side effects, although not everybody gets them.
The following are side effects that have been observed in patients taking Ventolin Disk, inhalation powder.
Side effects to look out for
Allergic reactions:These occur very rarely (in less than 1 in 10,000 patients taking the medicine).
Symptoms include:
- skin rash (hives) or redness,
- swelling, mainly of the face, lips, tongue, or throat (angioedema),
- worsening of wheezing, coughing, or difficulty breathing,
- sudden feeling of weakness or dizziness (which can lead to falls or loss of consciousness). The patient should contact their doctor immediately if they experience any of these symptoms. They should stop using Ventolin Disk.
The patient should contact their doctor immediately if they experience:
- a faster or stronger than usual heartbeat (palpitations). This is usually not serious and resolves soon after taking the medicine,
- an irregular heartbeat. The patient should not stop using Ventolin Disk unless their doctor advises them to do so.
Common side effects(occurring in less than 1 in 10 patients, but more than 1 in 100 patients taking the medicine):
- tremors,
- headache,
- rapid heartbeat.
Uncommon side effects(occurring in less than 1 in 100 patients, but more than 1 in 1,000 patients taking the medicine):
- palpitations,
- irritation of the mouth and throat mucosa,
- muscle cramps.
Rare side effects(occurring in less than 1 in 1,000 patients, but more than 1 in 10,000 patients taking the medicine):
- decreased potassium levels in the blood,
- peripheral vasodilation.
Very rare side effects(occurring in less than 1 in 10,000 patients taking the medicine):
- allergic reactions (see above),
- excessive restlessness,
- heart rhythm disorders,
- paradoxical bronchospasm. As with other inhaled medicines, paradoxical bronchospasm may occur, which is characterized by wheezing immediately after taking the medicine. In this situation, the patient should stop using Ventolin Disk, inhalation powder, and contact their doctor immediately. The doctor will assess the patient's condition and, if necessary, recommend alternative treatment.
Side effects with unknown frequency:
- myocardial ischemia, which may be indicated by, for example, chest pain.
If any side effect worsens or any side effects not listed in this leaflet occur, the patient should tell their doctor or pharmacist.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, the patient should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Post-Marketing Surveillance of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Reporting side effects helps to gather more information on the safety of the medicine.
5. How to store Ventolin Disk
- The medicine should be stored out of sight and reach of children.
- The medicine should not be used after the expiry date stated on the label or carton. The expiry date (EXP) is the last day of the month. Batch number (Lot).
- The medicine should be stored at a temperature not exceeding 30°C.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Ventolin Disk contains
- The active substance of the medicine is salbutamol in the form of salbutamol sulfate. Each dose (inhalation) of the medicine contains 200 micrograms of salbutamol in the form of salbutamol sulfate.
- The other ingredient is lactose monohydrate (which contains milk proteins).
What Ventolin Disk looks like and what the packaging contains
Inhalation powder.
Packaging:
The Disk inhaler device contains a foil strip with doses placed on it. The foil protects the inhalation powder from environmental conditions.
The Disk inhaler device, containing 60 doses of inhalation powder, with a mouthpiece and dose counter, in a carton.
Manufacturer:
Glaxo Wellcome Production
Zone Industrielle No.2
23, rue Lavoisier
27000 Evreux
France
To obtain more detailed information, the patient should contact the representative of the marketing authorization holder:
GSK Services Sp. z o.o.
ul. Rzymowskiego 53
02-697 Warsaw
tel. (22) 576-90-00
Date of last revision of the leaflet: January 2025
Marketing authorization holder:
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
D24 YK11
Ireland
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGlaxo Wellcome Production
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ventolin DiskDosage form: Aerosol, 100 mcg/dose inh.Active substance: salbutamolManufacturer: Laboratorio Aldo-Union S.A.Prescription requiredDosage form: Powder, 100 mcg/doseActive substance: salbutamolManufacturer: Orion CorporationPrescription requiredDosage form: Powder, 200 mcg/doseActive substance: salbutamolManufacturer: Orion CorporationPrescription required
Alternatives to Ventolin Disk in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ventolin Disk in Spain
Alternative to Ventolin Disk in Ukraine
Online doctors for Ventolin Disk
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ventolin Disk – subject to medical assessment and local rules.














