
Varilrix
Ask a doctor about a prescription for Varilrix

How to use Varilrix
Leaflet accompanying the packaging: information for the user
VARILRIX, powder and solvent for solution for injection in a pre-filled syringe
Syringe
Live varicella vaccine
Read the leaflet carefully before using the vaccine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed specifically for you. Do not pass it on to others.
- If you experience any side effects, including any not listed in this leaflet, please inform your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Varilrix vaccine and what is it used for
- 2. Important information before using Varilrix vaccine
- 3. How to use Varilrix vaccine
- 4. Possible side effects
- 5. How to store Varilrix vaccine
- 6. Contents of the packaging and other information
1. What is Varilrix vaccine and what is it used for
Varilrix vaccine is used in individuals from 12 months of age to prevent varicella. In certain circumstances, Varilrix vaccine may also be given to infants from 9 months of age.
How Varilrix vaccine works
After vaccination with Varilrix vaccine, the patient's immune system (the body's natural defense system) produces antibodies that protect against varicella virus infection.
2. Important information before using Varilrix vaccine
When not to use Varilrix vaccine
Warnings and precautions
Before administering Varilrix vaccine, discuss with your doctor, pharmacist, or nurse:
After or even before receiving any injection, fainting (especially in adolescents) may occur. Therefore, the patient should inform the doctor or nurse if they have ever fainted during an injection.
Varilrix vaccine and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, including those obtained without a prescription and vaccinations, as well as any medicines you plan to take.
Pregnancy, breastfeeding, and fertility
Varilrix vaccine should not be given to pregnant women.
3. How to use Varilrix vaccine
Varilrix vaccine should be administered subcutaneously or intramuscularly, in the upper arm or outer thigh.
Contraindications
- hypersensitivity to the active substance or to any of the excipients
- severe immunodeficiency
Special warnings and precautions for use
Varilrix vaccine should be used with caution in patients with a history of febrile seizures or family history of seizure disorders.
Interaction with other medicinal products and other forms of interaction
Varilrix vaccine may interact with other medicines, including immunosuppressants, salicylates, and antiviral agents.
Fertility, pregnancy and lactation
There are limited data on the use of Varilrix vaccine in pregnant women.
Effects on ability to drive and use machines
Varilrix vaccine has no or negligible influence on the ability to drive and use machines.
Undesirable effects
Like all medicines, Varilrix vaccine can cause side effects, although not everybody gets them.
Overdose
In the event of an overdose, the patient should be monitored for any adverse reactions and treated symptomatically.
5. How to store Varilrix vaccine
Store in a refrigerator (2°C - 8°C).
Shelf life
The shelf life of Varilrix vaccine is 24 months.
Special precautions for disposal
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
6. Contents of the packaging and other information
What Varilrix vaccine contains
- The active substance is live, attenuated varicella virus (Oka strain, grown in human diploid cell line MRC-5).
- The other ingredients are amino acids, lactose, sorbitol, and mannitol.
What Varilrix vaccine looks like and contents of the pack
Varilrix vaccine is a powder and solvent for solution for injection in a pre-filled syringe.
Marketing authorization holder and manufacturer
GlaxoSmithKline Biologicals SA, Rue de l'Institut 89, 1330 Rixensart, Belgium.
Date of the last update of the leaflet: May 2025
-------------------------------------------------------------------------------------------------------------------------
Information intended exclusively for professional medical personnel:
Similarly to all vaccines administered by injection, it is always necessary to ensure the possibility of appropriate treatment and medical supervision in the event of rare post-vaccination anaphylactic reactions.
Alcohol, as well as other disinfectants, should evaporate from the skin before administering the vaccine, as they can inactivate the attenuated viruses present in the vaccine.
Varilrix vaccines should never be administered intravascularly or intradermally.
This medicinal product should not be mixed with other medicinal products, as compatibility studies have not been performed.
The solvent and the vaccine after reconstitution should be visually inspected. Due to minor pH differences, the vaccine after reconstitution may have a color ranging from light peach to pinkish fuchsia. After reconstitution, semi-transparent particles of the product may be visible.
This is a normal phenomenon and does not affect the efficacy of the vaccine.
If the vaccine has a different color or contains other solid particles, it should not be administered.
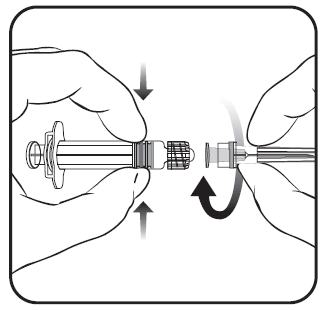
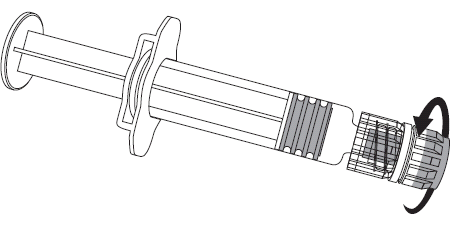
The vaccine should be reconstituted by adding the entire solvent from the ampoule to the vial with the powder. The vial should be shaken vigorously until the powder is completely dissolved.
The entire contents of the vial should be drawn back.
A new needle should be used for vaccine administration.
The vaccine should be administered immediately after reconstitution.
However, it has been shown that the reconstituted vaccine can be stored for up to 90 minutes at room temperature (25°C) or up to 8 hours in the refrigerator (at a temperature of 2°C – 8°C). If the reconstituted vaccine is not used within the recommended time or has not been stored under recommended conditions, it should be discarded.
Any unused medicinal product or waste should be disposed of in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGlaxoSmithKline Biologicals S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VarilrixDosage form: Powder, not less than 1350 PFU/0.5 mlActive substance: varicella, live attenuatedManufacturer: Merck Sharp & Dohme B.V.Prescription requiredDosage form: Suspension, 160 ELISA antigen units of hepatitis A virus, strain GBM/0.5 ml; 1 dose (0.5 ml)Active substance: hepatitis A, inactivated, whole virusPrescription requiredDosage form: Suspension, 60 mcg HA/strain, 1 dose (0.7 ml)Active substance: influenza, inactivated, split virus or surface antigenPrescription required
Alternatives to Varilrix in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Varilrix in Ukraine
Alternative to Varilrix in Spain
Online doctors for Varilrix
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Varilrix – subject to medical assessment and local rules.














