
Vagirux

Ask a doctor about a prescription for Vagirux

How to use Vagirux
Leaflet accompanying the packaging: information for the user
Vagirux, 10 micrograms, vaginal tablets
Estradiol
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any further questions, you should ask your doctor or pharmacist.
- This medicine has been prescribed for a specific person. It should not be given to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Vagirux and what is it used for
- 2. Important information before using Vagirux
- 3. How to use Vagirux
- 4. Possible side effects
- 5. How to store Vagirux
- 6. Contents of the packaging and other information
1. What is Vagirux and what is it used for
Vagirux contains estradiol.
- Estradiol is a female sex hormone.
- It belongs to a group of hormones called estrogens.
- It is identical to the estradiol produced by the ovaries in women.
Vagirux belongs to a group of medicines known as hormone replacement therapy (HRT) for vaginal administration.
Vagirux is used toalleviate symptoms of menopause that occur in the vagina, such as dryness or irritation. In medical terminology, this phenomenon is called "atrophic vaginitis". It is caused by a decrease in estrogen levels in the body and naturally occurs after menopause.
Vagirux works byreplacing estrogen, which is produced by the ovaries in women. The medicine is administered vaginally, and the hormone is released where it is needed. This can help alleviate discomfort in the vagina.
2. Important information before using Vagirux
Medical history and regular check-ups
Using HRT involves risks, which should be considered when the patient decides whether to use hormone replacement therapy or continue using it.
Experience in treating women in premature menopause (due to ovarian failure or surgery) is limited. If the patient is experiencing premature menopause, the risk associated with using HRT may differ. You should talk to your doctor.
Before starting (or resuming) HRT, the doctor should take a medical history, including a family history. The doctor may decide to perform an examination, such as a breast examination and/or gynecological examination, if necessary.
If the patient starts using Vagirux, they should consult their doctor at least once a year. During follow-up visits, they should discuss the benefits and risks of continuing to use Vagirux with their doctor.
Patients should regularly perform breast screening tests as recommended by their doctor.
When not to use Vagirux
If any of the following diseases or resulting doubts occur, you should talk to your doctorbefore starting to use Vagirux.
Do not use Vagirux if the patient:
- has been diagnosed with, has had, or is suspected of having breast cancer;
- has been diagnosed with, has had, or is suspected of having estrogen-dependent tumors, such as endometrial cancer (cancer of the uterine lining);
- has unexplained vaginal bleeding;
- has excessive thickening of the uterine lining(endometrial hyperplasia) that has not been treated;
- has been diagnosed with or has had blood clots in the veins(thrombosis), such as deep vein thrombosis or pulmonary embolism;
- has a blood clotting disorder(such as protein C, protein S, or antithrombin deficiency);
- has had a disease caused by blood clots in the arteries, such as heart attack, stroke, or angina pectoris;
- has been diagnosed with or has had liver diseaseand liver function tests have not returned to normal;
- has been diagnosed with a rare blood disorder - porphyria, which is inherited.
- has an allergyto estradiolor any of the other ingredients of Vagirux (listed in section 6).
If any of the above diseases occur for the first time while using Vagirux, you should stop using it and contact your doctor as soon as possible.
Warnings and precautions
Before starting treatment, you should inform your doctor if you currently have or have had any of the following diseases, as they may recur or worsen while using Vagirux. In such cases, your doctor may consider more frequent check-ups.
- Uterine fibroids;
- Endometriosis (growth of uterine lining outside the uterus) or excessive growth of uterine lining (endometrial hyperplasia) in the past;
- Increased risk of blood clots (see "Blood clots in the veins (thrombosis)");
- Increased risk of estrogen-dependent tumors (breast cancer in the mother, sister, or grandmother);
- High blood pressure;
- Liver disease, such as a benign liver tumor;
- Diabetes;
- Gallstones;
- Migraine or severe headaches;
- Systemic lupus erythematosus (a disease that affects many organs);
- Epilepsy;
- Asthma;
- Otosclerosis (a disease that affects the eardrum and hearing);
- Very high levels of fats (triglycerides) in the blood;
- Fluid retention due to impaired heart or kidney function;
- Hereditary and acquired angioedema.
You should stop using Vagirux and contact your doctor immediatelyif any of the following conditions occur while using HRT:
- Any of the diseases listed in the "When not to use Vagirux" section above;
- Yellowing of the skin and eyes (jaundice), which may be symptoms of liver disease;
- Swelling of the face, tongue, and/or throat, and/or difficulty swallowing or hives with difficulty breathing, which may indicate angioedema;
- Significant increase in blood pressure (symptoms include headache, fatigue, and dizziness);
- Migraine headache that occurs for the first time;
- If the patient becomes pregnant;
- If the patient experiences symptoms of blood clots, such as:
- painful swelling and redness of the legs,
- sudden chest pain,
- difficulty breathing.
Note:Vagirux is not a contraceptive. If it has been less than 12 months since the last menstrual period or the patient is under 50 years old, it may be necessary to use an additional method of contraception. You should consult your doctor.
HRT and cancer
Excessive thickening of the uterine lining (endometrial hyperplasia) and uterine lining cancer (endometrial cancer)
Long-term use of HRT in the form of tablets containing only estrogen may increase the risk of developing uterine lining cancer (endometrium).
It is not known whether a similar risk exists during repeated or prolonged (longer than one year) use of Vagirux. However, Vagirux is absorbed into the bloodstream to a very small extent, and therefore, the addition of progestogen is not necessary.
Bleedingor spottingis usually not a cause for concern, but you should consult your doctor. It may be a sign of endometrial thickening.
The described risks apply to HRT medicines that are absorbed into the bloodstream. Vagirux is used locally in the vagina, and its absorption into the bloodstream is very small. It is less likely that the mentioned disorders will worsen or recur while using Vagirux, but if you have any doubts, you should consult your doctor.
Breast cancer
Data indicate that using Vagirux does not increase the risk of breast cancer in women who have never had it before. It is not known whether Vagirux can be safely used in women who have had breast cancer.
You should regularly examine your breasts. You should contact your doctor if you notice any of the following changes:
- indentation of the skin,
- changes in the nipple,
- presence of lumps that are visible or palpable.
In addition, it is recommended to perform screening mammography tests as recommended by your doctor.
Ovarian cancer
Ovarian cancer is rare - much rarer than breast cancer. Using HRT that only contains estrogen is associated with a slightly increased risk of ovarian cancer.
Comparison
The risk of ovarian cancer depends on age. For example, in women aged 50-54 who do not use HRT, ovarian cancer will be diagnosed within 5 years in about 2 out of 2000 women. In women who have taken HRT for 5 years, it occurs in about 3 out of 2000 women (i.e., about 1 additional case).
Effect of HRT on the heart and circulation
Blood clots in the veins (thrombosis)
The risk of developing blood clots in the veinsis 1.3 to 3 times higher in women using HRT compared to those not using it, especially in the first year of use.
The formation of blood clots can have serious consequences, and if they move to the lungs, they can cause chest pain, shortness of breath, loss of consciousness, or even death.
The risk of blood clots in the veins is higher if the patient is older or if any of the following situations apply to the patient. You should inform your doctor if any of the following situations apply to you:
- the patient is unable to walk for a long time due to a serious operation, injury, or illness (see also section 3, "If an operation is planned");
- the patient has significant obesity (BMI > 30 kg/m2);
- there are thromboembolic disorders that require long-term use of medications to prevent blood clots;
- the patient or a close family member has had blood clots in the legs, lungs, or other organs in the past;
- the patient has systemic lupus erythematosus;
- the patient has been diagnosed with cancer.
If symptoms of blood clots occur, see "You should stop using Vagirux and contact your doctor immediately".
Comparison
In women aged 50-59 who do not use HRT, the number of cases of blood clots in the veins within 5 years is estimated to be 4 to 7 per 1000 women.
In women aged 50-59 who have used estrogen-only HRT for more than 5 years, the number of cases will be 5 to 8 per 1000 (i.e., 1 additional case).
Heart disease (heart attack)
In women using estrogen-only HRT, there is no increased risk of developing heart disease.
Stroke
The risk of stroke is about 1.5 times higher in women using HRT compared to those not using it. The number of additional stroke cases caused by HRT increases with age.
Comparison
In women aged 50-59 who do not use HRT, the number of stroke cases within 5 years is estimated to be 8 per 1000 women. In women aged 50-59 who use HRT, the number of cases within 5 years will be 11 per 1000 women (i.e., 3 additional cases).
Other conditions
HRT does not prevent memory loss. The risk of probable memory loss may be slightly higher in women who start using HRT at an age over 65. You should consult your doctor.
Vagirux and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, including those that are available without a prescription, herbal medicines, or other natural products.
The likelihood of interactions with other medicines is low, as Vagirux is used locally in the vagina. Vagirux may affect other local treatments used in the vagina.
Pregnancy and breastfeeding
Vagirux is intended for use only in postmenopausal women. If the patient becomes pregnant, they should stop using the medicine and contact their doctor.
Driving and using machines
It is not known whether Vagirux affects the ability to drive or use machines.
3. How to use Vagirux
This medicine should always be used as directed by your doctor. If you are unsure, you should consult your doctor or pharmacist.
Using the medicine
- Vagirux can be started on any day.
- For vaginal use only. Do not take the tablets orally.
- The vaginal tablet should be inserted into the vagina using the applicator.
The "INSTRUCTIONS FOR USE" at the end of this leaflet contain detailed instructions. Before using Vagirux, you should read the instructions carefully.
The applicator for vaginal tablets is intended for multiple use up to 24 times for one patient (one tablet per application). Then, the applicator should be discarded with household waste. Do not use applicators that show visible signs of damage.
What dose to use
- For the first 2 weeks, use one vaginal tablet once a day.
- Then, use one vaginal tablet twice a week. There should be a 3-4 day interval between doses.
General information about treating menopausal symptoms
- Your doctor will determine the smallest effective dose of Vagirux for the shortest possible period. You should consult your doctor if the recommended dose seems too high or too low.
- Treatment should be continued only if the benefits outweigh the risks. You should discuss this with your doctor.
Using a higher dose of Vagirux than recommended
- If a higher dose of Vagirux is taken than recommended, you should contact your doctor or pharmacist.
- Vagirux is intended for local use in the vagina. The dose of estradiol is so small that a large number of tablets would be needed to achieve the dose usually used for oral treatment.
Missing a dose of Vagirux
- If a dose is missed, you should use the missed tablet as soon as possible.
- Do not use a double dose to make up for the missed dose.
Stopping the use of Vagirux
You should not stop using Vagirux without consulting your doctor. Your doctor will explain the consequences of stopping treatment and discuss other possible treatment methods.
If an operation is planned
If the patient is scheduled to undergo surgery, they should inform the surgeon that they are using Vagirux. It may be necessary to stop using Vagirux 4-6 weeks before the operation to reduce the risk of blood clots (see section 2, "Blood clots in the veins (thrombosis)").
Before resuming the use of Vagirux, you should consult your doctor.
If you have any further doubts about using this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Vagirux can cause side effects, although not everybody gets them.
In women using HRT in the form of medicines that are absorbed into the bloodstream, the following diseases have been reported more frequently compared to women not using HRT. The following risks are less likely to apply to medicines used locally in the vagina, such as Vagirux:
- ovarian cancer;
- blood clots in the veins of the legs or lungs (venous thromboembolism);
- stroke;
- probable memory loss, if HRT is started at an age over 65.
For more information, see section 2, "Important information before using Vagirux".
Common: may affect up to 1 in 10 women
- headache;
- abdominal pain;
- vaginal bleeding, discharge, or discomfort.
Uncommon: may affect up to 1 in 100 women
- genital fungal infections;
- nausea;
- rash;
- weight gain;
- hot flashes;
- high blood pressure.
Rare: may affect up to 1 in 10,000 women
- diarrhea;
- fluid retention;
- worsening of migraine;
- generalized hypersensitivity (e.g., anaphylactic reaction/anaphylactic shock).
During treatment with systemic estrogens, the following side effects have been reported:
- gallstones;
- various skin disorders:
- skin discoloration, especially on the face or neck, known as melasma (chloasma);
- painful red skin nodules (erythema nodosum);
- rash with characteristic redness or pain (erythema multiforme).
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych,
Al. Jerozolimskie 181C,
02-222 Warszawa,
tel.: + 48 22 49 21 301,
faks: + 48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Vagirux
The medicine should be stored out of sight and reach of children.
The blisters should be stored in the outer packaging to protect them from light.
Do not use this medicine after the expiry date stated on the carton and blister after "Expiry date". The expiry date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment. This medicine may pose a risk to aquatic environments.
6. Contents of the packaging and other information
What Vagirux contains
- The active substance of Vagirux is estradiol. Each vaginal tablet contains estradiol hemihydrate, equivalent to 10 micrograms of estradiol.
- The other ingredients are: hypromellose 15 mPa·s, lactose monohydrate, cornstarch, and magnesium stearate. Tablet coating: hypromellose 5 mPa·s and macrogol 6000.
What Vagirux looks like and contents of the packaging
The vaginal tablets are white, round, and coated with the letter "E" embossed on one side, with a diameter of about 6 mm.
Pack sizes:
18 vaginal tablets in a blister pack with a separately packaged vaginal applicator for multiple use with LDPE/PP in a protective foil with PP.
The whole thing is in a cardboard box.
24 vaginal tablets in a blister pack with a separately packaged vaginal applicator for multiple use with LDPE/PP in a protective foil with PP.
The whole thing is in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
GEDEON RICHTER POLSKA Sp. z o.o.
ul. Ks. J. Poniatowskiego 5
05-825 Grodzisk Mazowiecki
Polska
Manufacturer
Haupt Pharma Münster GmbH
Schleebrüggenkamp 15
48159 Münster
Niemcy
Gedeon Richter Plc.
Gyömrői út 19-21.
Budapeszt 1103
Węgry
To obtain more detailed information on the medicine, you should contact:
GEDEON RICHTER POLSKA Sp. z o.o.
Medical Department
ul. ks. J. Poniatowskiego 5
05-825 Grodzisk Mazowiecki
tel. +48 (22)755 96 48
[email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Węgry
Vagirux 10 mikrogramm hüvelytabletta
Austria
Rewellfem 10 Mikrogramm Vaginaltabletten
Lichtenstein
Rewellfem
Czechy
Vagirux
Słowacja
Vagirux 10 mikrogramov vaginálne tablety
Dania
Rewellfem
Islandia
Rewellfem
Norwegia
Vagirux
Finlandia
Vagirux
Szwecja
Vagirux
Estonia
VAGIRUX
Łotwa
Vagirux 10 mikrogrami vaginālās tabletes
Litwa
VAGIRUX 10 mikrogramų makšties tabletės
Chorwacja
Vagirux 10 mikrograma tablete za rodnicu
Słowenia
Vagirux 10 mikrogramov vaginalne tablete
Irlandia
Vagirux
Malta
Vagirux 10 microgram vaginal tablets
Hiszpania
Vagirux 10 microgram vaginal tablets
Włochy
Vagirux
Polska
Vagirux
Portugalia
Formyra
Rumunia
ESTRADIOL RICHTER 10 micrograme comprimate
vaginale
Date of last revision of the leaflet: April 2022
INSTRUCTIONS FOR USE
How to use Vagirux
- 1. Remove the protective foil from the applicator. Open it from the side shown in the picture.
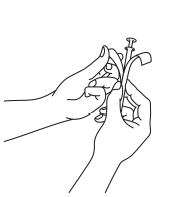
- 2. Holding the tube, pull the plunger of the applicator until it stops. Remove one vaginal tablet from a separate blister pack and insert it firmly into the holder (the wide end) of the applicator tube.
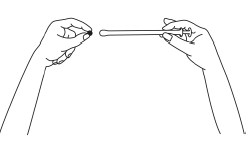
- 3. Carefully insert the applicator into the vagina until you feel resistance (8-10 cm).
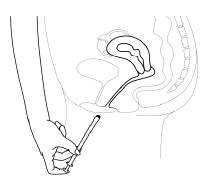
- 4. To release the tablet, press the plunger until you feel resistance. The tablet will stick to the vaginal wall immediately. The tablet will not fall out when standing or walking.
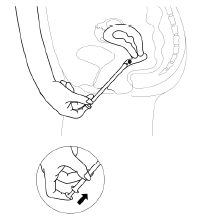
- 5. After each use, before the next use, you should wash the applicator according to the following cleaning instructions:
- Remove the plunger from the applicator.
- Clean both the tube and the plunger using mild soap and rinse thoroughly with warm tap water. Rinse both the inner and outer surfaces of the tube.
- If necessary, remove excess water from both the tube and the plunger by shaking them briefly.
- Let the tube and plunger air dry on a clean surface (e.g., a paper towel).
- Reinsert the plunger into the applicator tube for later use.
- 6. The applicator should be used until the packaging is empty (18 or 24 times), and then it should be discarded with household waste. Do not use applicators that show visible signs of damage.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGedeon Richter Plc. Haupt Pharma Muenster GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VagiruxDosage form: Gel, 0.5 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: Gel, 1 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: Gel, 1 mg/gActive substance: estradiolPrescription required
Alternatives to Vagirux in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Vagirux in España
Alternative to Vagirux in Ucrania
Online doctors for Vagirux
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Vagirux – subject to medical assessment and local rules.










