
Vagifem

Ask a doctor about a prescription for Vagifem

How to use Vagifem
Leaflet accompanying the packaging: information for the user
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
Vagifem, 10 micrograms, vaginal tablets
Estradiol
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4, "Possible side effects".
Table of contents of the leaflet
- 1. What is Vagifem and what is it used for
- 2. Important information before using Vagifem
- 3. How to use Vagifem
- 4. Possible side effects
- 5. How to store Vagifem
- 6. Contents of the packaging and other information
1. What is Vagifem and what is it used for
Vagifem contains estradiol
- Estradiol is a female sex hormone.
- It belongs to a group of hormones called estrogens.
- It is identical to the estradiol produced by the ovaries in women.
Vagifem belongs to a group of medicines known as hormone replacement therapy (HRT) for vaginal administration.
It is usedto alleviate symptoms of menopause that occur in the vagina, such as dryness or irritation. In medical terminology, this phenomenon is called "atrophic vaginitis". It is caused by a decrease in estrogen levels in the body and naturally occurs after menopause.
Vagifem worksby replacing estrogen, which is produced by the ovaries in women.
The medicine is administered vaginally, and the hormone is released where it is needed. This can help alleviate discomfort in the vagina.
Experience in treating women over 65 years of age is limited.
2. Important information before using Vagifem
Medical history and regular check-ups
Using HRT involves risks that should be considered when the patient decides to use hormone replacement therapy or continue using it.
Experience in treating women in premature menopause (due to ovarian failure or surgery) is limited. If the patient is experiencing premature menopause, the risk associated with HRT may be different. You should talk to your doctor.
Before starting (or resuming) HRT, the doctor should take a medical history, including a family history. The doctor may decide to perform an examination, such as a breast examination and/or gynecological examination, if necessary.
If the patient starts using Vagifem, they should consult their doctor at least once a year. During follow-up visits, they should discuss the benefits and risks of continuing to use Vagifem with their doctor.
Patients should regularly perform breast screening tests in accordance with their doctor's recommendations.
When not to use Vagifem
In the event of any of the following diseases or resulting doubts, you should consult your doctorbefore starting to use Vagifem.
Do not use Vagifem if the patient:
- has been diagnosed with allergy(hypersensitivity) to estradiolor any of the other ingredients of Vagifem (listed in section 6, "Contents of the packaging and other information");
- has been diagnosed with, has had, or is suspected of having breast cancer;
- has been diagnosed with, has had, or is suspected of having estrogen-dependent tumors, such as endometrial cancer (cancer of the uterine lining);
- has been diagnosed with unexplained vaginal bleeding;
- has been diagnosed with excessive thickening of the uterine lining(endometrial hyperplasia), which has not been treated;
- has been diagnosed with or has had blood clots in the veins(thrombosis), such as in the legs (deep vein thrombosis) or lungs (pulmonary embolism);
- has a blood clotting disorder(such as protein C, protein S, or antithrombin deficiency);
- has had or recently had a disease caused by blood clots, such as heart attack, stroke, or angina pectoris;
- has been diagnosed with or has had liver diseaseand liver test results have not returned to normal;
- has been diagnosed with a rare blood disorder - porphyria, which is inherited.
If any of the above diseases occur for the first time while using Vagifem, you should stop using it immediately and contact your doctor as soon as possible.
Warnings and precautions
Before starting treatment, you should inform your doctor if you currently have or have had any of the following diseases, as they may recur or worsen during the use of Vagifem. In such cases, your doctor may decide that more frequent check-ups are necessary.
- asthma;
- epilepsy;
- diabetes;
- gallstones;
- high blood pressure;
- migraine or severe headaches;
- liver disease, such as a benign liver tumor;
- endometriosis (growth of uterine lining outside the uterus) or excessive growth of the uterine lining (endometrial hyperplasia) in the past;
- a disease affecting the eardrum and hearing - otosclerosis;
- a disease of the immune system that affects many organs - systemic lupus erythematosus;
- increased risk of estrogen-dependent tumors (breast cancer in the mother, sister, or grandmother);
- increased risk of blood clots (see "Blood clots in the veins (venous thromboembolism)");
- uterine fibroids;
- very high levels of fats (triglycerides) in the blood;
- fluid retention due to impaired heart or kidney function;
- hereditary and acquired angioedema.
You should stop using Vagifem and contact your doctor immediatelyif you experience any of the following conditions while using HRT:
- migraine headache that occurred for the first time;
- yellowing of the skin and eyes (jaundice), which may be symptoms of liver disease;
- swelling of the face, tongue, and/or throat, or difficulty swallowing or hives, along with difficulty breathing, which may indicate angioedema;
- significant increase in blood pressure (symptoms include headache, fatigue, and dizziness);
- occurrence of any of the diseases listed in the "When not to use Vagifem" section above;
- if the patient is pregnant;
- if the patient experiences symptoms of blood clots, such as:
- painful swelling and redness of the legs,
- sudden chest pain,
- difficulty breathing.
Note: Vagifem is not a contraceptive. If it has been less than 12 months since the last menstrual period or the patient is under 50 years of age, additional contraceptive methods may be necessary. You should consult your doctor.
HRT and tumors
Excessive thickening of the uterine lining (endometrial hyperplasia) and uterine lining cancer (endometrial cancer)
Long-term use of HRT tablets containing only estrogen may increase the risk of developing uterine lining cancer (endometrium).
It is not known whether a similar risk exists during repeated or prolonged (longer than one year) use of Vagifem. However, Vagifem is absorbed into the bloodstream to a very small extent, and therefore, the addition of progestogen is not necessary.
Bleedingor spottingis not usually a cause for concern, but you should consult your doctor. It may be a sign of endometrial hyperplasia.
The risks described below are related to HRT medicines that circulate in the blood. Vagifem, on the other hand, is intended for local use in the vagina and is absorbed into the bloodstream to a very small extent. The worsening or recurrence of the disorders mentioned below during the use of Vagifem is less likely, but if you have any concerns, you should consult your doctor.
Breast cancer
Data indicate that using Vagifem does not increase the risk of breast cancer in women who have never had it before. It is not known whether Vagifem can be safely used in women who have had breast cancer.
You should regularly examine your breasts. You should contact your doctor if you notice any of the following changes:
- indentation of the skin,
- changes in the nipple,
- presence of lumps that are visible or palpable.
In addition, it is recommended to perform screening mammograms in accordance with your doctor's recommendations.
Ovarian cancer
Ovarian cancer is rare - much rarer than breast cancer. The use of HRT that only contains estrogen is associated with a slightly increased risk of ovarian cancer.
Comparison
The risk of ovarian cancer depends on age. For example, in women between the ages of 50 and 54 who do not use HRT, ovarian cancer will be diagnosed within 5 years in about 2 out of 2000 women. In women who have taken HRT for 5 years, it will occur in about 3 out of 2000 women (i.e., about 1 additional case).
Effect of HRT on the heart and circulation
Blood clots in the veins (venous thromboembolism)
The risk of developing blood clots in the veinsis 1.3 to 3 times higher in women using HRT compared to those not using it, especially in the first year of use.
The formation of blood clots can have serious consequences, and if they move to the lungs, they can cause chest pain, shortness of breath, loss of consciousness, or even death.
The risk of blood clots in the veins is higher if the patient is older or if any of the following situations apply to the patient. You should inform your doctor if any of the following situations apply to you:
- the patient is unable to walk for a long time due to a serious operation, injury, or illness (see also section 3, "If an operation is planned");
- the patient has significant obesity (BMI > 30 kg/m2);
- the patient has thromboembolic disorders that require long-term use of medications to prevent blood clots;
- the patient or a close family member has had blood clots in the legs, lungs, or other organs in the past;
- the patient has systemic lupus erythematosus;
- the patient has been diagnosed with cancer.
If you experience symptoms of blood clots, see "You should stop using Vagifem and contact your doctor immediately".
Comparison
In women between the ages of 50 and 59 who do not use HRT, the number of cases of blood clots in the veins within 5 years is estimated to be 4 to 7 per 1000 women.
In women between the ages of 50 and 59 who use estrogen-only HRT for more than 5 years, the number of cases will be 5 to 8 per 1000 (i.e., 1 additional case).
Heart disease (heart attack)
In women using estrogen-only HRT, there is no increased risk of developing heart disease.
Stroke
The risk of stroke is about 1.5 times higher in women using HRT compared to those not using it. The number of additional stroke cases caused by HRT increases with age.
Comparison
In women between the ages of 50 and 59 who do not use HRT, the number of stroke cases within 5 years is estimated to be 8 per 1000 women on average. In women between the ages of 50 and 59 who use HRT, the number of cases within 5 years will be 11 per 1000 women (i.e., 3 additional cases).
Other conditions
HRT does not prevent memory loss. The risk of possible memory loss may be slightly higher in women who start using HRT at an age over 65. You should consult your doctor.
Vagifem and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, including those that are available without a prescription, herbal medicines, or other natural products. The likelihood of interactions with other medicines is low, as Vagifem is used locally in the vagina. Vagifem may affect other local treatments used in the vagina.
Pregnancy and breastfeeding
Vagifem is intended for use only in postmenopausal women. If the patient becomes pregnant, they should stop using the medicine and contact their doctor.
Driving and using machines
It is not known whether Vagifem affects the ability to drive or use machines.
3. How to use Vagifem
This medicine should always be used in accordance with the doctor's recommendations. If you have any doubts, you should consult your doctor or pharmacist.
Using the medicine
- Vagifem can be started on any day.
- The vaginal tablet should be inserted into the vagina using the applicator.
The "INSTRUCTIONS FOR USE" at the end of this leaflet contain detailed instructions. Before using Vagifem, you should read the instructions carefully.
What dose to use
- For the first 2 weeks, one vaginal tablet should be used once a day.
- Then, one vaginal tablet should be used twice a week. The interval between doses should be 3-4 days.
General information about treating menopausal symptoms
- The doctor will determine the smallest effective dose of Vagifem for the shortest possible period. You should talk to your doctor if the recommended dose seems too high or insufficient.
- Treatment should be continued only if the benefits outweigh the risks. You should discuss this with your doctor.
Using a higher dose of Vagifem than recommended
- In the event of taking a higher dose of Vagifem than recommended, you should contact your doctor or pharmacist.
- Vagifem is intended for local use in the vagina. The dose of estradiol is so small that you would need to use a large number of tablets to achieve the dose usually used for oral treatment.
Missing a dose of Vagifem
- If a dose is missed, you should use the missed tablet as soon as possible.
- You should not use a double dose to make up for the missed dose.
Stopping the use of Vagifem
You should not stop using Vagifem without consulting your doctor. Your doctor will explain the consequences of stopping treatment and discuss other possible treatment options.
If an operation is planned
If the patient is scheduled to undergo surgery, they should inform the surgeon that they are using Vagifem. It may be necessary to stop using Vagifem 4 to 6 weeks before the operation to reduce the risk of blood clots (see section 2, "Blood clots in the veins (venous thromboembolism)").
Before resuming the use of Vagifem, you should consult your doctor.
If you have any further doubts about the use of this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Vagifem can cause side effects, although not everybody gets them.
The following diseases are reported more frequently in women using HRT medicines that circulate in the blood than in women not using HRT. This risk is less likely to apply to medicines administered vaginally, such as Vagifem:
- ovarian cancer;
- blood clots in the veins of the legs or lungs (venous thromboembolism);
- stroke;
- possible memory loss, if HRT is started at an age over 65. For more information, see section 2, "Important information before using Vagifem".
Common: may affect less than 1 in 10 women
- headache,
- abdominal pain,
- vaginal bleeding, discharge, or palpable discomfort.
Uncommon: may affect less than 1 in 100 women
- genital fungal infections,
- feeling unwell (nausea),
- rash,
- weight gain,
- hot flashes,
- high blood pressure.
Rare: may affect less than 1 in 10,000 women
- diarrhea,
- fluid retention,
- worsening of migraine,
- generalized hypersensitivity (e.g., anaphylactic reaction/anaphylactic shock).
During the use of systemic estrogens, the following side effects have been reported:
- gallstones,
- various skin disorders:
- skin discoloration, especially on the face or neck, known as pregnancy spots (chloasma);
- painful red skin nodules (erythema nodosum);
- rash with characteristic redness or pain (erythema multiforme).
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Adverse Reaction Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help gather more information on the safety of the medicine.
5. How to store Vagifem
The medicine should be stored out of sight and reach of children.
Do not store in the refrigerator.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
This medicine may pose a risk to the aquatic environment.
6. Contents of the packaging and other information
What Vagifem contains
- The active substance of Vagifem is estradiol 10 micrograms (in the form of estradiol hemihydrate). Each vaginal tablet contains 10 micrograms of estradiol (in the form of estradiol hemihydrate).
- Vagifem also contains: hypromellose, lactose monohydrate, cornstarch, and magnesium stearate.
- The ingredients of the tablet coating are: hypromellose and macrogol 6000.
What Vagifem looks like and what the packaging contains
Each white vaginal tablet is placed in a single-dose applicator.
The Vagifem tablet has the marking NOVO 278 on one side.
Pack sizes:
18 vaginal tablets with applicators.
24 vaginal tablets with applicators.
For more detailed information, you should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Greece, the country of export:
Νονο Nordisk Hellas Ltd
Panagouli 80 & Agias Triados 65
153 43 Agia Paraskevi
Greece
Manufacturer:
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Marketing authorization number in Greece, the country of export:54165/6-7-2016
Parallel import authorization number:350/24
Date of leaflet approval: 01.10.2024
[Information about the trademark]
INSTRUCTIONS FOR USE
How to use Vagifem
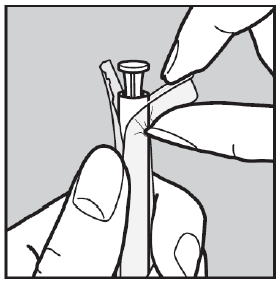
- 1. Open a single blister package from the side shown in the picture.
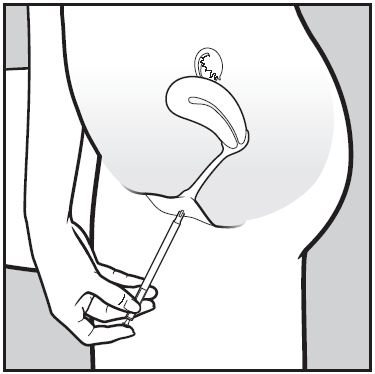
- 2. Carefully insert the applicator into the vagina until you feel resistance (8-10 cm).
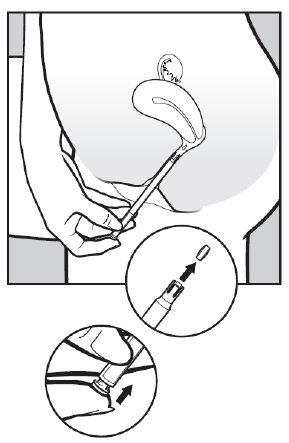
- 3. To release the tablet, gently press the plunger until you hear a click. The tablet will immediately adhere to the vaginal wall. The tablet will not fall out when standing or walking.
- 4. Remove the applicator and discard it in the trash.
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Νονο Nordisk Hellas Ltd
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VagifemDosage form: Gel, 0.5 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: Gel, 1 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: Gel, 1 mg/gActive substance: estradiolPrescription required
Alternatives to Vagifem in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Vagifem in Spain
Alternative to Vagifem in Ukraine
Online doctors for Vagifem
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Vagifem – subject to medical assessment and local rules.










