

True Test 36

Ask a doctor about a prescription for True Test 36

How to use True Test 36
PATIENT INFORMATION LEAFLET
Leaflet attached to the packaging: information for the user
TRUE Test 36, Patch Test
TRUE Test 36 consists of 3 panels - self-adhesive patches. Each panel contains 12 patches, totaling 36 patches. 35 patches are coated with a layer containing test substances. One patch (patch number 9) is an empty patch.
Active substance
micrograms/cm
micrograms/patch
Panel No. 1
- 1. Nickel sulfate 200 162
- 2. Lanolin alcohols 1000 810
- 3. Neomycin sulfate 600 486
- 4. Potassium dichromate 54 44
- 5. Kain mixture 630 510
- 6. Fragrance mixture 430 348
- 7. Colophony 1200 972
- 8. Paraben mixture 1000 810
- 9. Empty patch
- 10. Peru balsam 800 648
- 11. Ethylenediamine dihydrochloride 50 41
- 12. Cobalt chloride 20 16
Panel No. 2
- 13. p-tert-Butylphenol-formaldehyde resin 45 36
- 14. Epoxy resin 50 41
- 15. Carbon black mixture 250 203
- 16. Black rubber mixture 75 61
- 17. Cl+Me-isothiazolinone 4 3
- 18. Quaternium-15 100 81
- 19. Methyldibromoglutaronitrile 5.0 4.1
- 20. Paraphenylenediamine 80 65
- 21. Formaldehyde 180 146
- 22. Mercaptan mixture 75 61
- 23. Thimerosal 7 6
- 24. Thiuram mixture 27 22
Panel No. 3
- 25. Diazolidinyl urea 550 450
- 26. Quinoline mixture 190 154
- 27. Tixocortol pivalate 3.0 2.4
- 28. Gold sodium thiosulfate 75 61
- 29. Imidazolidinyl urea 600 490
- 30. Budesonide 1.0 0.81
- 31. Hydrocortisone 17-butyrate 20 16
- 32. Mercaptobenzothiazole 75 61
- 33. Bacitracin 600 490
- 34. Parthenolide 3.0 2.4
- 35. Blue pigment 106 50 41
- 36. 2-Bromo-2-nitropropane-1,3-diol 250 200
a) Five parts benzocaine and one part each of cinchocaine hydrochloride and tetracaine hydrochloride.
b) Five parts geraniol and oak moss extract, four parts hydroxy citronellal and cinnamic alcohol, two parts cinnamic aldehyde and eugenol, and one part each of isoeugenol and amylcinnamic aldehyde.
c) Methylparaben, ethylparaben, propylparaben, butylparaben, and benzylparaben in equal weight proportions.
d) Diphenylguanidine, zinc diethyldithiocarbamate, and zinc dibutyldithiocarbamate in equal weight proportions.
e) Two parts N-isopropyl-N'-phenyl-paraphenylenediamine, five parts N-cyclohexyl-N'-phenyl-paraphenylenediamine, and five parts N,N'-diphenyl-paraphenylenediamine.
f) Currently, the preparation contains N-hydroxymethyl imide of succinic acid.
g) Morpholinyl mercaptobenzothiazole and N-cyclohexylbenzothiazylsulfonamide and dibenzothiazyl disulfide in equal weight proportions.
h) Disulfiram, dipentamethylenethiuram disulfide, tetramethylthiuram disulfide, and tetramethylthiuram sulfide in equal weight proportions.
i) Clioquinol and chlorquinaldol in equal weight proportions.
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again. If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is TRUE Test 36 and what is it used for
- 2. Important information before using TRUE Test 36
- 3. How to use TRUE Test 36
- 4. Possible side effects
- 5. How to store TRUE Test 36
- 6. Contents of the pack and other information
1. What is TRUE Test 36 and what is it used for
TRUE Test 36 is used for the diagnosis of allergic contact dermatitis. Contact dermatitis is a skin reaction to foreign substances that cause an allergic reaction.
TRUE Test 36 is a ready-to-use patch test for determining the cause of allergic contact dermatitis.
TRUE Test 36 is intended for use in adults.
The test consists of 3 self-adhesive patches containing 12 patches each. Each patch contains a test substance that can cause a skin reaction in people with hypersensitivity. Such substances are called allergens. Each patch contains a different allergen, and one empty patch does not contain any allergen. TRUE Test 36 contains 35 of the most common allergens/allergen mixtures, and one empty patch.
TRUE Test 36 is performed to confirm hypersensitivity to any of the test substances (allergens) that make up the panels. If the substance to which the patient is hypersensitive comes into contact with the skin, it will cause an inflammatory reaction called contact dermatitis.
These substances can be components of perfumes or aftershave, ointments or creams, rubber gloves, industrial chemicals, etc. The substances in TRUE Test 36 are known allergens. If the patient is hypersensitive to a specific substance contained in the patch, the skin reaction under the patch will be redness and inflammation. If the patient is not hypersensitive to a specific substance in the patch, there will be no skin reaction. It is possible to be hypersensitive to more than one test substance.
2. Important information before using TRUE Test 36
When not to use TRUE Test 36
- If there is severe or generalized dermatitis. The test should be postponed until the exacerbation of symptoms subsides.
- If the patient is hypersensitive to any of the other components of TRUE Test 36 (listed in section 6).
Warnings and precautions
- Avoid exposing the test area to sunlight. Sunburn can cause false-negative reactions to allergens that the patient is hypersensitive to.
- Avoid excessive sweating while wearing the test patches.
- If the patient is taking medications that suppress the immune system, such as corticosteroids (e.g., prednisolone) or corticosteroid creams/ointments (e.g., hydrocortisone). Do not use them for at least two weeks before starting the test.
- In cases of "angry back" syndrome (a condition where there is hypersensitivity caused by dermatitis in another part of the body). If there is a reaction to all test patches, it may be necessary to repeat the test on another day.
- In patients who have previously experienced anaphylactoid reactions, the use of TRUE Test 36 should be carefully considered.
Consult a doctor before using TRUE Test 36 if any of the above factors apply to the patient. The doctor will decide on the appropriate course of action.
Hypersensitivity: In rare cases, hypersensitivity to substances present in the patches may occur during the use of TRUE Test 36. The occurrence of a reaction later than 10 days after application of the test may indicate contact hypersensitivity.
TRUE Test 36 should only be applied to skin that does not have:
- acne
- scars
- inflammatory foci
- changes that may affect the test results. In case of doubt, consult a doctor.
Protect the test application site from moisture. During bathing or showering, avoid getting the patch or surrounding skin wet. If the patch gets wet, it may come off, allowing the test substances to be washed away.
Avoid situations such as sunbathing or physical exercise that may cause the patch to come off.
Butylhydroxyanisole (BHA) (E320) and butylhydroxytoluene (BHT) (E321) are present in patch No. 7 Colophony (panel 1) to preserve its stability. BHA and BHT may cause local skin reactions (e.g., contact dermatitis), which may affect the occurrence of a false-positive reaction to Colophony.
Children
TRUE Test 36 is not recommended for use in children, as its safety and efficacy in these patients have not been established.
TRUE Test 36 and other medicines
Before applying TRUE Test 36, tell your doctor or pharmacist about all medicines the patient is currently taking or has recently taken, including those obtained without a prescription. Remember that the dermatologist may not be aware of other medicines being taken.
At least 2 weeks before the test, stop using local or oral corticosteroids if the daily dose is equivalent to or exceeds 20 mg of prednisolone, as corticosteroids may suppress a positive test reaction.
Pregnancy, breastfeeding, and fertility
It is not recommended to use TRUE Test 36 in pregnant women. If the patient is pregnant or thinks she may be pregnant, she should consult a doctor before using TRUE Test 36.
Driving and using machines
It is unlikely that the use of TRUE Test 36 will affect the ability to drive or operate machinery. In case of doubt, consult a doctor.
3. How to use TRUE Test 36
TRUE Test 36 is applied by a doctor.
- 1. Open the packaging and remove the TRUE Test 36 panel.
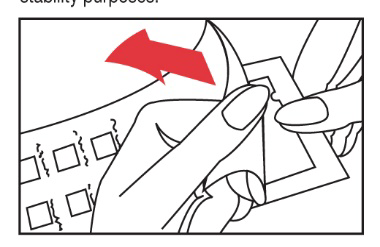
- 2. Remove the protective foil from the panel surface. Be careful not to touch the test substances. The packaging of panel 2 contains a desiccant that ensures the stability of the allergens.
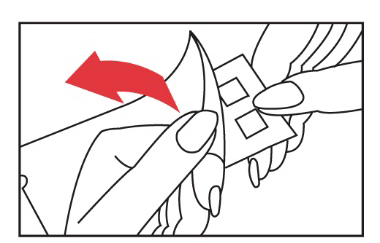
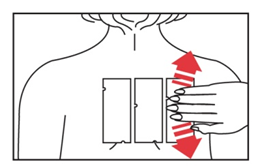
- 3. Apply the test to the patient's back - or the outer part of the arm. Gently smooth the patch surface from the center to the edges to ensure good contact between the individual allergens and the skin. Both patches are applied on either side, a few centimeters away from the spine. The third panel is placed next to one of the remaining panels.
to ensure good contact between the individual allergens and the skin. Both patches are applied on either side, a few centimeters away from the spine. The third panel is placed next to one of the remaining panels.
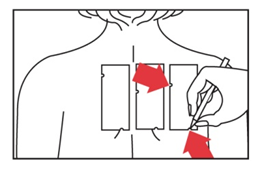
- 4. Use a special medical marker to mark the skin at the location corresponding to the two notches on each patch.
TRUE Test 36 should remain on the skin for 48 hours. Do not remove or move it.
Make sure it does not get wet (water, sweat).
If the patch is removed or comes off prematurely, it is possible that positive skin reactions to allergens that the patient is actually hypersensitive to may not occur. If the patch is removed or comes off before 48 hours, consult a doctor.
After 48 hours, the patient or doctor can remove the patches.
When to read the results?
The doctor should read the test results half an hour after removing the test and again 1-2 days after its removal, when allergic reactions have fully developed and mild skin irritations have subsided.
Certain allergens may sometimes cause reactions that appear later than 4-5 days after the test is removed. In such cases, inform the doctor.
What will the doctor look for?
The doctor should carefully examine the test area for signs of an allergic reaction. This reaction usually occurs as a rash with swelling, redness, and small blisters. Redness alone does not necessarily indicate an allergic reaction. If an allergy occurs, the doctor will provide the following information:
- where the patient is likely to come into contact with irritating substances on a daily basis,
- how to best avoid these substances. The doctor may suggest alternative substances to those that should be avoided.
In case of doubt, consult a doctor or pharmacist.
In case of discomfort in the test areas, contact a doctor.
The doctor may decide to remove the test.
4. Possible side effects
Like all medicines, TRUE Test 36 can cause side effects, although not everybody gets them.
Very common side effects (may affect more than 1 in 10 people) include:
- irritation caused by the patch, which usually resolves quickly, burning of the skin, prolonged test reactions. A positive reaction to the test usually disappears within 1-2 weeks, while a prolonged test reaction may persist for weeks or months.
Common side effects (may affect up to 1 in 10 people) include:
- transient discoloration or hyperpigmentation of the skin due to the test reaction.
- redness of the skin caused by irritation or inflammation (erythema).
Uncommon side effects (may affect up to 1 in 100 people) include:
- exacerbation of skin inflammatory changes
Rare side effects (may affect up to 1 in 1000 people) include:
- hypersensitivity to the substance on the test panel.
Frequency not known (frequency cannot be estimated from the available data):
- anaphylactic reaction (systemic reaction, with possible life-threatening hypotension).
- hypersensitivity (allergic reaction).
In very rare cases, and only in relation to specific substances, anaphylactic reactions (systemic reactions with possible life-threatening hypotension) have occurred. Allergy clinics are prepared to treat such cases for other reasons. No anaphylactic reactions have been reported after the use of TRUE Test 36.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocides
Al. Jerozolimskie 181C
PL-02 222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store TRUE Test 36
Store in a refrigerator (2°C - 8°C).
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date (EXP) is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What TRUE Test 36 contains
In addition to the active substances listed on the first page, the test contains the following excipients: polyester fiber patch and adhesive (ethylene and vinyl acetate copolymer) with acrylic adhesive, polyester patches, povidone 90, hydroxypropyl cellulose, methylcellulose, beta-cyclodextrin, sodium carbonate, sodium bicarbonate, butylhydroxyanisole, and butylhydroxytoluene.
What TRUE Test 36 looks like and what the pack contains
Each panel is covered with a silicone-coated polyethylene foil and packed in a multi-layered packaging.
The packaging of panel 2 contains a desiccant that ensures the stability of the allergens during storage.
Pack contents: 10 sets (1 set = one panel No. 1, one panel No. 2, and one panel No.
- 3).
Marketing authorization holder and manufacturer
SmartPractice Denmark ApS
Herredsvejen 2
3400 Hillerød
Denmark
[email protected]
Representative in Poland:
BENEMEDO Sp. z o.o.
ul. Floriańska 2
03-707 Warsaw
Date of last revision of the leaflet:
24-02-2021
------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
A template is attached to each packaging of TRUE Test 36, allowing for quick identification of the allergen that caused the reaction. To ensure correct reading, the marks on the skin should correspond to the notches on the template. Note that side 1 of the template corresponds to panel No. 1, and side 2 of the template corresponds to panel No. 2.
Interpretation of results recommended by the International Contact Dermatitis Research Group:
- negative reaction
?
questionable reaction; slight spotted erythema, minimal infiltration or no infiltration
+
weak positive reaction (non-bullous); erythema, slight infiltration; papules may occur
++
strong positive reaction (bullous); erythema, infiltration, papules, vesicles
+++
very strong positive reaction; intense erythema, infiltration, coalescing vesicles
IR
irritation reactions of various types
NT
not tested
Note
- Patients who have been found to have no reaction may be hypersensitive to other substances not included in this set. Furthermore, false-negative reactions may occur. Repeating the test or testing with additional substances may be indicated.
- A positive reaction should meet the criteria for an allergic reaction (papular or vesicular erythema and infiltration).
- The presence of pustules, scattered papules, or uniform erythematous changes without infiltration usually indicates irritation and does not suggest an allergic reaction.
In evaluating a positive reaction, it is not the number of "+" signs in the severity scale of the reaction to the test substances that matters, but rather the confirmation of the allergic nature of the reaction (as opposed to a non-specific irritation reaction).
Some allergens (neomycin sulfate, paraphenylenediamine, lanolin alcohols, kain mixture, gold sodium thiosulfate, parthenolide, blue pigment 106, bacitracin, imidazolidinyl urea, diazolidinyl urea, budesonide, hydrocortisone 17-butyrate, tixocortol pivalate) may sometimes cause reactions that only become apparent 4-5 days after the test is applied. Patients should be informed about this. A delayed reaction should be verified during an additional visit to the doctor on day 5-7.
All positive reactions should be carefully evaluated, taking into account the patient's medical history and symptoms, especially in the case of positive reactions to specific allergens with lower relevant sensitization rates (e.g., gold sodium thiosulfate).
Contraindications
Severe or generalized dermatitis. The test should be postponed until the acute symptoms of the disease subside.
Hypersensitivity to any of the excipients, in addition to the active substances.
Special warnings and precautions for use
Hypersensitivity to a substance on the test panel occurs only in rare cases. A reaction in response to the test that appears on day 10 or later may indicate contact hypersensitivity.
"Angry back" syndrome is a condition where there is a reaction caused by dermatitis in another part of the body or by a strong allergic reaction to the test allergens. Therefore, be cautious when evaluating test results in patients who have multiple positive test results for individual test substances.
To verify false-positive results, it may be necessary to repeat the test after a certain period.
In patients who have previously experienced anaphylactoid reactions, the use of TRUE Test 36 should be carefully considered.
Avoid excessive sweating and sun exposure at the test site. Sunburn can reduce the reactivity of the test and cause false-negative results.
The doctor should not apply the patch to the skin in areas with acne, scars, inflammatory foci, or other changes that may affect the test results.
In the event of a strong reaction to the patch test, local corticosteroids can be applied. In rare cases, it may be necessary to use systemic corticosteroids.
Butylhydroxyanisole (BHA) (E320) and butylhydroxytoluene (BHT) (E321) are present as antioxidants in patch No. 7 Colophony (panel 1). BHA and BHT may cause local skin reactions (e.g., contact dermatitis), which may affect the occurrence of a false-positive reaction to Colophony.
- Country of registration
- Prescription requiredNo
- Manufacturer
- ImporterSmartpractice Demnark ApS
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to True Test 36Dosage form: Solution, 40 mg/5 mlActive substance: indigo carmineManufacturer: SERB SERB SAPrescription not requiredDosage form: Capsules, 250 mgActive substance: metyraponePrescription requiredDosage form: Solution, 40 mg/5 mlActive substance: indigo carmineManufacturer: CenexiPrescription not required
Alternatives to True Test 36 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to True Test 36 in Spain
Alternative to True Test 36 in Ukraine
Online doctors for True Test 36
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for True Test 36 – subject to medical assessment and local rules.














