
Transtec 70 mcg/h

Ask a doctor about a prescription for Transtec 70 mcg/h

How to use Transtec 70 mcg/h
Package Leaflet: Information for the Patient
Transtec 35 μg/h, 20 mg, Transdermal System, Patch
Transtec 52.5 μg/h, 30 mg, Transdermal System, Patch
Transtec 70 μg/h, 40 mg, Transdermal System, Patch Buprenorphine
You should carefully read the contents of this leaflet before using the medicine, as it contains important information for you.
- You should keep this leaflet, so you can read it again later.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- 1. What is Transtec 35 μg/h, Transtec 52.5 μg/h, and Transtec 70 μg/h, and what is it used for
- 2. Important information before using Transtec 35 μg/h, Transtec 52.5 μg/h, and Transtec 70 μg/h
- 3. How to use Transtec 35 μg/h, Transtec 52.5 μg/h, and Transtec 70 μg/h
- 4. Possible side effects
- 5. How to store Transtec 35 μg/h, Transtec 52.5 μg/h, and Transtec 70 μg/h
- 6. Contents of the pack and other information
1.
What is Transtec 35 μg/h, Transtec 52.5 μg/h, and Transtec 70 μg/h, and what is it used for
Transtec is a pain-relieving medicine used to treat moderate to severe pain in cancer patients and severe pain in other patients that has not responded to other non-opioid pain relievers. Transtec works through the skin. After applying the transdermal system, the active substance (buprenorphine) is released and absorbed through the skin into the bloodstream. Buprenorphine is an opioid (strong pain reliever) that reduces pain by acting on the central nervous system (specialized nerve cells in the spinal cord and brain). The pain-relieving effect lasts for up to 4 days. Transtec is not suitable for treating acute (short-term) pain.
2. Important information before using Transtec 35 μg/h, Transtec 52.5 μg/h, and Transtec 70 μg/h
When not to use Transtec
- if you are allergic to buprenorphine or any of the other ingredients of this medicine (listed in section 6);
- in patients addicted to opioids;
- in existing or threatened respiratory failure;
- in patients treated with MAO inhibitors (medicines used to treat depression) and 2 weeks after stopping MAO inhibitors (see "Transtec and other medicines");
- if you have been diagnosed with muscle weakness (myasthenia gravis);
- if you experience alcohol withdrawal syndrome (delirium tremens - a state of confusion, tremors caused by alcohol withdrawal in addicted individuals or occurring during a drinking binge);
- if you are pregnant;
Do not use Transtec to treat withdrawal symptoms in individuals addicted to drugs.
Warnings and precautions
Tolerance, dependence, and addiction
This medicine contains buprenorphine, which is an opioid. Repeated use of opioids can lead to tolerance (the medicine becoming less effective). Long-term use of Transtec can also lead to dependence, abuse, and addiction, which can be life-threatening. The risk of these side effects may increase with higher doses and longer treatment duration.
- if you or a family member have ever abused or been dependent on alcohol, prescription drugs, or illegal substances ("addiction");
- if you are a smoker;
- if you have ever had mood problems (depression, anxiety, or personality disorders) or have been treated by a psychiatrist for other mental health conditions.
If you experience any of the following symptoms while taking Transtec, it may indicate dependence:
- needing to take the medicine for longer than prescribed by your doctor
- needing to take a higher dose than prescribed
- continuing to take the medicine even if it no longer relieves pain
- using the medicine for reasons other than prescribed, such as "to relax" or "to sleep".
- frequently attempting to stop or control the use of the medicine but being unable to do so.
- experiencing withdrawal symptoms after stopping the medicine, such as feeling unwell, and then feeling better after taking the medicine again ("withdrawal symptoms").
If you experience any of these symptoms, talk to your doctor to discuss the best treatment option for you, including when to stop taking the medicine and how to do it safely (see section 3, Stopping Transtec). Before starting Transtec, discuss it with your doctor, pharmacist, or nurse.
- with acute alcohol intoxication;
- with seizure attacks;
- with consciousness disorders of unknown etiology;
- in shock (which may be characterized by cold sweats);
- with increased intracranial pressure and no possibility of assisted ventilation;
- with respiratory disorders or taking medicines that may inhibit the respiratory center (see "Transtec and other medicines");
- with depression or other conditions treated with antidepressant medicines.
- Using these medicines together with Transtec may lead to serotonin syndrome, a life-threatening condition (see "Transtec and other medicines").
- with liver function disorders.
Also, take the following precautions:
- Fever and high environmental temperature can increase the absorption of the medicine through the skin and disrupt the proper adhesion of the transdermal system to the skin. Therefore, avoid exposure to high external temperatures (such as saunas, infrared radiation, electric blankets, hot water bottles) and consult your doctor if you have a high fever.
Athletes should be aware that this medicine may cause a positive result in doping tests.
Sleep apnea
Transtec may cause sleep apnea, such as pauses in breathing during sleep, and hypoxemia (low oxygen levels in the blood). Symptoms may include pauses in breathing during sleep, nighttime awakenings due to shortness of breath, difficulty staying asleep, or excessive daytime sleepiness. If you or someone else notices these symptoms, contact your doctor. Your doctor may consider reducing the dose.
Children and adolescents
Do not use Transtec in patients under 18 years of age due to insufficient studies in this patient group.
Transtec and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or might take.
- Do not take Transtec with MAO inhibitors (medicines used to treat depression), also within 2 weeks before starting Transtec.
- You may experience drowsiness, illness, weakness, or slower and shallower breathing. These symptoms may worsen if you take other strong pain relievers (opioids), certain sedatives, or medicines used to treat certain mental health conditions, such as tranquilizers, antidepressants, neuroleptics, gabapentin, or pregabalin used to treat epilepsy or nerve pain (neuropathic pain).
Tell your doctor or pharmacist if you are taking:
- medicines used to treat allergies, motion sickness, or nausea (antihistamines or antiemetics)
- medicines used to treat mental health conditions (antipsychotics or neuroleptics)
- muscle relaxants;
- medicines used to treat Parkinson's disease;
Taking Transtec with sedatives, such as benzodiazepines or related medicines, increases the risk of drowsiness, breathing difficulties (respiratory depression), coma, and can be life-threatening. Therefore, taking such medicines together should only be considered when other treatment options are not possible.
- If your doctor prescribes Transtec with sedatives, they will use a limited dose and duration of concomitant treatment with these medicines.
- Tell your doctor about all sedatives you are taking and follow your doctor's dosage instructions carefully. It may be helpful to inform friends or relatives about the possibility of these symptoms. If they occur, contact your doctor.
- If you take Transtec with certain medicines, the effect of the transdermal system may be enhanced. These medicines include certain antibacterial and antifungal medicines (such as those containing erythromycin and ketoconazole) and those used to treat HIV (such as those containing ritonavir).
- If you take Transtec with certain medicines, the effect of the transdermal system may be reduced. These medicines include dexamethasone, antiepileptic medicines (such as those containing carbamazepine, phenytoin), and antitubercular medicines (such as rifampicin).
- Certain medicines may enhance the side effects of Transtec and sometimes cause severe reactions. While taking Transtec, do not take other medicines without consulting your doctor, especially antidepressants, such as citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, duloxetine, venlafaxine, amitriptyline, doxepin, or trimipramine. These medicines may interact with Transtec and cause symptoms such as involuntary, rhythmic muscle contractions, including those affecting eye movement, agitation, hallucinations, coma, excessive sweating, tremors, increased reflexes, muscle tension, and body temperature above 38°C. If you experience these symptoms, contact your doctor.
Transtec with food, drink, and alcohol
Do not drink alcohol while using Transtec. Alcohol may enhance certain side effects of buprenorphine and cause discomfort.
Drinking grapefruit juice at the same time may enhance the effect of Transtec.
Pregnancy, breastfeeding, and fertility
If you are pregnant, breastfeeding, think you may be pregnant, or plan to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
There is insufficient experience with the use of Transtec in pregnant women.
Using Transtec during pregnancy is contraindicated.
Buprenorphine, the active substance of Transtec, inhibits lactation and passes into breast milk.
Do not use Transtec while breastfeeding.
Driving and using machines
Transtec may cause dizziness, drowsiness, blurred or double vision, and affect your reactions, making it difficult to participate in traffic and operate machinery.
This is especially true:
- at the start of treatment;
- when changing the dose;
- after stopping another pain reliever and switching to Transtec;
- when taking other centrally acting medicines;
- when drinking alcohol. In these cases, do not drive or operate machinery while using Transtec. This also applies when stopping Transtec. Patients using Transtec should not drive or operate machinery for at least 24 hours after removing the transdermal system.
If you are unsure, consult your doctor or pharmacist.
3. How to use Transtec 35 μg/h, Transtec 52.5 μg/h, Transtec 70 μg/h
Always use this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Before starting treatment and regularly during treatment, your doctor will discuss with you what to expect from Transtec, when and how long to take it, when to contact your doctor, and when to stop the medicine (see also "Stopping Transtec").
Transtec transdermal system is available in 3 strengths: Transtec 35 micrograms/hour, Transtec 52.5 micrograms/hour, and Transtec 70 micrograms/hour.
The choice of which strength of Transtec to use is made by your doctor. During treatment, if necessary, your doctor may change the strength of the medicine to a lower or higher one.
The recommended dose of Transtec is:
Adults
If your doctor has not prescribed otherwise, one Transtec transdermal system should be applied to the skin (as described below) and replaced no later than every 4 days. For convenience, you can change it twice a week on the same days of the week, e.g., always on Mondays and Thursdays. To remind you to change the transdermal system, mark the day of the week on the calendar on the outer packaging. If your doctor has prescribed an additional pain reliever, follow your doctor's instructions carefully to get the full benefit from Transtec.
Children and adolescents
Do not use Transtec in patients under 18 years of age due to insufficient studies in this patient group.
Elderly patients (over 65 years)
No dose adjustment is necessary for these patients.
Patients with kidney problems
No dose adjustment is necessary for patients with renal failure.
Patients with liver problems
The strength and duration of action of Transtec may change in patients with liver function disorders. Your doctor will closely monitor patients with liver failure.
How to use
Before applying the transdermal system, patch
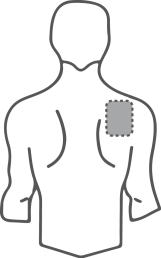
- Choose a flat, clean, and hairless area of skin on the upper part of the body, preferably the upper chest or upper back (see illustrations).
Potential areas on the body for applying the transdermal system, patch
Chest
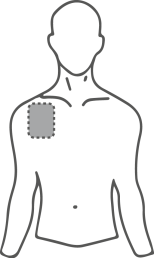
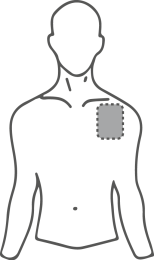
or
Back
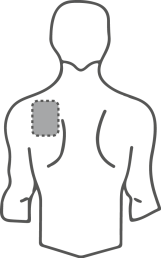
If you have difficulty applying the transdermal system, ask for help.
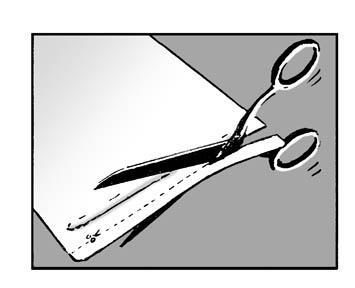
- If the chosen skin area is hairy, remove the hair with scissors. Do not shave!
- Avoid areas of skin that are red, irritated, or have other lesions, such as extensive scars.
- The chosen skin area should be dry and clean. If the skin needs to be cleaned before applying the preparation, wash it with cold or lukewarm water. Do not use soap or any other cleaning agent. After a hot bath or shower, wait until the skin is completely dry and cool. Avoid using skin care products (cosmetic milks, creams, ointments) on the skin areas where the transdermal system will be applied, as this may impair its adhesion.
Applying the transdermal system
Step 1:
Each individual transdermal system is in a pouch. Cut the child-resistant pouch along the dotted line using scissors. Be careful not to damage the patch.
or
Remove the patch from the pouch.
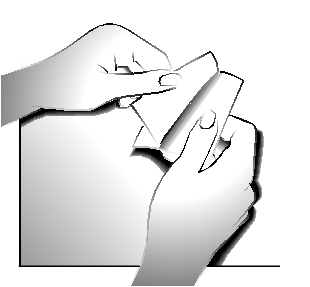
Step 2:
The adhesive layer of the transdermal system is covered with a silver protective foil. Carefully separate halfof the protective foil. Try not to touch the adhesive layer of the transdermal system.

Step 3:
Place the transdermal system on the chosen skin area and remove the remaining protective foil.
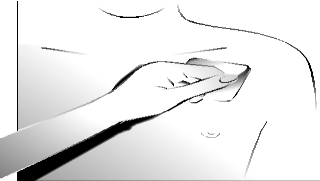
Step 4:
Press firmly with your hand and hold for about 30 seconds. Make sure the entire transdermal system adheres to the skin, especially the edges.
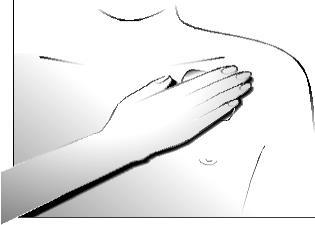
Wearing the transdermal system
You can wear the Transtec transdermal system for up to 4 days. After proper application, the risk of the transdermal system detaching is low. While wearing the transdermal system, you can bathe, shower, and swim. Do not expose it to excessive heat from the outside (such as saunas, infrared radiation, electric blankets, hot water bottles).
If the transdermal system detaches prematurely (very unlikely), do not reuse it. Apply a new transdermal system (see "Changing the transdermal system" below).
Changing the transdermal system
- Fold the patch in half with the adhesive side inward.
- Dispose of it in a place invisible and inaccessible to children.
- Apply subsequent patches to different skin areas (as described above). You can apply a transdermal system to the same area of skin again after at least one week.
Duration of use
Your doctor will determine the duration of use of Transtec. Do not stop treatment on your own, as pain and discomfort may return (see also "Stopping Transtec").
If you feel that the effect of Transtec is too strong or too weak, talk to your doctor or pharmacist.
Using a higher dose of Transtec than prescribed
You may experience symptoms of buprenorphine overdose.
Overdose may enhance the side effects of buprenorphine, such as drowsiness, nausea, and vomiting. Pupils may become pinpoint, breathing may become slow and shallow, and circulatory collapse may occur.
If you realize you have taken a higher dose than prescribed, remove the transdermal system immediately and contact your doctor or pharmacist.
Missing a dose of Transtec
If you are taking the medicine according to a fixed schedule and forget to take it on time, take the prescribed dose as soon as possible. This may change the days of the week, e.g., from Mondays and Thursdays to Wednesdays and Saturdays. Mark this change on the outer packaging of the patches. If you miss a dose by a large margin, pain may return. In this case, contact your doctor.
Do not take a double dose to make up for a missed dose.
Stopping Transtec
After stopping or ending treatment with Transtec too early, pain may return.
If the reason for stopping is unpleasant side effects, consult your doctor before stopping. Your doctor will determine what to do and assess the possibility of changing treatment.
In some individuals, after stopping long-term treatment with strong pain relievers, withdrawal symptoms may occur. The risk of withdrawal symptoms after Transtec is low. However, if you experience agitation, anxiety, nervousness, shivers, excessive restlessness, difficulty sleeping, or digestive disorders, inform your doctor about these symptoms.
If you have any further questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Transtec can cause side effects, although not everybody gets them.
If you experience swelling of the hands, feet, ankles, face, lips, mouth, or throat, causing difficulty swallowing or breathing, hives, weakness, or yellowing of the skin and eyes (jaundice), remove the transdermal system and contact your doctor immediately or go to the emergency department of the nearest hospital. These may be symptoms of a very rare but serious allergic reaction.
In some cases, delayed allergic reactions with marked inflammation have been reported. In such cases, after consulting your doctor, stop using Transtec.
The following side effects have been reported:
If you experience any of the following side effects, contact your doctor immediately.
Very common side effects(may affect more than 1 in 10 people):
nausea
redness, itching
Common side effects(may affect up to 1 in 10 people):
dizziness, headaches
shortness of breath
vomiting, constipation
skin changes (rash, usually with repeated use), excessive sweating
swelling (e.g., swelling of the legs), feeling tired
Uncommon side effects(may affect up to 1 in 100 people):
confusion, sleep disorders, restlessness
excessive sedation of varying degrees, from fatigue to lethargy
circulatory disorders (such as hypotension or rarely even circulatory collapse)
dry mouth
pruritus
fatigue
urinary disorders, urinary retention (less urine than usual)
Rare side effects(may affect up to 1 in 1,000 people):
loss of appetite
psychotic symptoms (such as hallucinations, anxiety, nightmares), decreased libido
difficulty concentrating, speech disorders, fatigue, balance disorders, abnormal skin sensations (numbness, tingling, or burning sensation)
vision disorders, blurred vision, eyelid swelling
hot flashes
breathing difficulties (respiratory depression)
heartburn
erectile dysfunction
hives
withdrawal syndrome (see below), reactions at the site of application of the transdermal system
Very rare side effects(may affect less than 1 in 10,000 people):
severe allergic reactions (see above)
addiction, sudden mood changes
muscle twitching, abnormal taste sensation
pupil constriction to the size of a pinhead
ear pain
abnormal breathing, hiccups
vomiting reflex
pustules, small blisters
chest pain
Frequency not known(frequency cannot be estimated from the available data):
contact dermatitis (skin rash with inflammation, which may include a burning sensation)
skin discoloration
In some individuals, after stopping long-term treatment with strong pain relievers, withdrawal symptoms may occur. The risk of withdrawal symptoms after Transtec is low. However, if you experience agitation, anxiety, nervousness, shivers, excessive restlessness, difficulty sleeping, or digestive disorders, inform your doctor about these symptoms.
Reporting side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. By reporting side effects, you can help provide more information on the safety of this medicine.
Side effects can also be reported to the marketing authorization holder.
5. How to store Transtec 35 μg/h, Transtec 52.5 μg/h, Transtec 70 μg/h
Keep this medicine out of the sight and reach of children. It may harm them, even if their symptoms are the same as yours.
Store in a place invisible and inaccessible to children.
Do not use this medicine after the expiry date stated on the carton and pouch after "Expiry date (month/year)". The expiry date refers to the last day of that month.
No special precautions for storage are necessary.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Transtec contains
The active substance of Transtec is buprenorphine.
| Transtec 35 micrograms/hour transdermal system, patch | 1 transdermal system, patch contains 20 mg buprenorphine (Buprenorphine) and releases approximately 35 micrograms of buprenorphine per hour. The surface area of the transdermal system containing the active substance is 25 cm2. |
| Transtec 52.5 micrograms/hour transdermal system, patch | 1 transdermal system, patch contains 30 mg buprenorphine (Buprenorphine) and releases approximately 52.5 micrograms of buprenorphine per hour. The surface area of the transdermal system containing the active substance is 37.5 cm2. |
| Transtec 70 micrograms/hour transdermal system, patch | 1 transdermal system, patch contains 40 mg buprenorphine (Buprenorphine) and releases approximately 70 micrograms of buprenorphine per hour. The surface area of the transdermal system containing the active substance is 50 cm2. |
Other ingredients are:
Adhesive matrix (containing buprenorphine): oleate (Z)-octadeca-9-en-1-yl, povidone K90, 4-oxopentanoic acid, cross-linked poly(acrylic acid-co-butyl acrylate-co-2-ethylhexyl acrylate-co-vinyl acetate), in a ratio of 5:15:75:5.
Adhesive matrix (not containing buprenorphine): non-cross-linked poly(acrylic acid-co-butyl acrylate-co-2-ethylhexyl acrylate-co-vinyl acetate), in a ratio of 5:15:75:5.
Separator foil between the adhesive matrices with and without buprenorphine:
foil made of poly(ethylene terephthalate).
Outer coating layer:
poly(ethylene terephthalate).
Removable protective layer covering the adhesive surface of the transdermal system containing buprenorphine – siliconized foil made of poly(ethylene terephthalate), one-sidedly aluminum-coated.
What Transtec looks like and contents of the pack
The transdermal systems, patches are flesh-colored with rounded edges and have printing:
Transtec 35 μg/h, Buprenorphine, 20 mg
Transtec 52.5 μg/h, Buprenorphine, 30 mg
Transtec 70 μg/h, Buprenorphine, 40 mg
Packaging contains 3, 5, and 10 patches packaged individually in child-resistant pouches.
Marketing authorization holder and manufacturer
Grünenthal GmbH
Zieglerstrasse 6
52078 Aachen
Germany
Date of last revision of the leaflet:11/2024
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGruenenthal GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Transtec 70 mcg/hDosage form: Solution, 0.3 mg/mlActive substance: buprenorphinePrescription requiredDosage form: Tablets, 0.2 mgActive substance: buprenorphinePrescription requiredDosage form: Tablets, 0.4 mgActive substance: buprenorphinePrescription required
Alternatives to Transtec 70 mcg/h in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Transtec 70 mcg/h in Spain
Alternative to Transtec 70 mcg/h in Ukraine
Online doctors for Transtec 70 mcg/h
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Transtec 70 mcg/h – subject to medical assessment and local rules.














