
Tipifree Combi
Ask a doctor about a prescription for Tipifree Combi

How to use Tipifree Combi
Package Leaflet: Information for the Patient
Tipifree Combi, 40 micrograms/ml + 5 mg/ml, eye drops, solution
Travoprost + Timolol
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Tipifree Combi and what is it used for
- 2. Important information before using Tipifree Combi
- 3. How to use Tipifree Combi
- 4. Possible side effects
- 5. How to store Tipifree Combi
- 6. Contents of the pack and other information
1. What is Tipifree Combi and what is it used for
Tipifree Combi eye drops is a combination of two active substances (travoprost and timolol). Travoprost is a prostaglandin analogue that works by increasing the outflow of aqueous fluid from the eye, which reduces its pressure. Timolol is a beta-blocker that works by reducing the production of fluid inside the eye. Both of these substances work together to reduce the pressure inside the eye.
Tipifree Combi in the form of eye drops is used to treat high pressure in the eye in adults, including the elderly. This pressure can lead to the development of a disease called glaucoma.
2. Important information before using Tipifree Combi
When not to use Tipifree Combi
Warnings and precautions
Before starting to use Tipifree Combi, discuss with your doctor if the following medical conditions are present or have been present:
- coronary heart disease (symptoms may include chest pain or feeling of pressure in the chest, difficulty breathing, shortness of breath), heart failure, low blood pressure;
- heart rhythm disorders, such as slow heart rate;
- breathing difficulties, asthma, or chronic obstructive pulmonary disease;
- diseases with impaired blood circulation (such as Raynaud's disease or Raynaud's syndrome);
- diabetes or tendency to spontaneous hypoglycemia (as timolol may mask symptoms of hypoglycemia - low blood sugar levels);
- hyperthyroidism (as timolol may mask objective and subjective symptoms of thyroid disease);
- myasthenia (chronic neuromuscular weakness);
- cataract surgery;
- eye inflammation, corneal disease.
If you are going to have any surgical procedure, inform your doctor that you are using Tipifree Combi, as timolol may affect the action of certain anesthetics.
During the administration of drugs that reduce the production of aqueous humor (e.g., timolol, acetazolamide), cases of detachment of the choroid after filtration surgery have been reported.
If the patient experiences any severe allergic reaction (skin rash, redness, and itching of the eye) while using Tipifree Combi, treatment with adrenaline may not be effective enough. Therefore, it is essential to inform your doctor that you are using Tipifree Combi if you are to receive any other treatment.
Tipifree Combi may change the color of the iris (the colored part of the eye); this change may be permanent.
Tipifree Combi may increase the length, thickness, color, and/or number of eyelashes and may cause abnormal hair growth on the eyelids.
Travoprost may be absorbed through the skin, and therefore, should not be used by pregnant or breastfeeding women. If any amount of the medicine gets on the skin, it should be washed off immediately.
Children and adolescents
Tipifree Combi is not intended for use in children and adolescents under 18 years of age.
Tipifree Combi and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or might take, including those obtained without a prescription.
Tipifree Combi may affect the action of other medicines taken at the same time or other medicines may affect the action of Tipifree Combi; this also applies to other medicines used to treat glaucoma.
Inform your doctor about taking or planning to take other medicines that lower blood pressure, used to treat heart diseases (including quinidine, used to treat heart diseases and some forms of malaria), antidiabetic drugs, or antidepressants, such as fluoxetine or paroxetine.
It is possible to have an additive effect, resulting in low blood pressure and/or significant slowing of the heart rate, when beta-adrenergic blockers in the form of eye drops are used together with medicines used for heart diseases (such as calcium channel blockers, beta-adrenergic receptor blockers, antiarrhythmic agents, including amiodarone, digitalis glycosides, parasympathomimetics, or guanethidine).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
Do not use Tipifree Combi if you are pregnant, unless your doctor considers it necessary. If you can become pregnant, use appropriate contraceptive methods while using the medicine.
Do not use Tipifree Combi if you are breastfeeding. Tipifree Combi may pass into breast milk.
Driving and using machines
After using Tipifree Combi, vision may be blurred for a while. Tipifree Combi may also cause hallucinations, dizziness, nervousness, or fatigue in some patients.
Do not drive or operate machinery until these symptoms have resolved.
Tipifree Combi contains macrogolglycerol hydroxystearate 40 and propylene glycol (E 1520)
The medicine may cause skin reactions and skin irritation.
3. How to use Tipifree Combi
This medicine should always be used exactly as your doctor has instructed. If you are not sure, ask your doctor or pharmacist.
The recommended dose is one drop into the conjunctival sac of the affected eye(s) once daily, in the morning or evening. Use the medicine every day at the same time.
Tipifree Combi can be used in both eyes only if your doctor has recommended it.
Tipifree Combi is for use as eye drops only.
If you are using other eye drops besides Tipifree Combi, wait at least 5 minutes between using Tipifree Combi and the other eye drops.
If you wear soft contact lenses, do not use Tipifree Combi while wearing the lenses. After using the medicine, wait 15 minutes before putting the lenses back in.
Method of administration
Follow the instructions below to use Tipifree Combi properly.
- 1. Wash your hands and sit or stand comfortably.
- 2. Remove the protective cap (Figure 1).
- 3. Hold the bottle upside down with your thumb on the cap and your other fingers on the bottom of the bottle (Figure 2). Before the first use, press the bottle several times, about 13 times, until the first drop appears.
- 4. Tilt your head back and gently pull down the lower eyelid to create a pocket between your eye and eyelid.
- 5. Place the tip of the bottle close to your eye without touching it.
- 6.
Do not touch the dropper to the eye, eyelid, or surrounding areas, as this may cause infection.
- 7. Hold the bottle as described above (Figure 2) in a vertical position over your eye and gently press the pump mechanism to release one drop into your eye, then release the lower eyelid (Figure 3).
- 8. Press your finger to the corner of your affected eye near your nose (Figure 4). Hold for 2 minutes, keeping your eye closed.
- 9. Administer the medicine to the other eye, if your doctor has recommended it.
- 10. Immediately after use, replace the cap on the bottle tip.
- 11. If a drop misses your eye, try again.
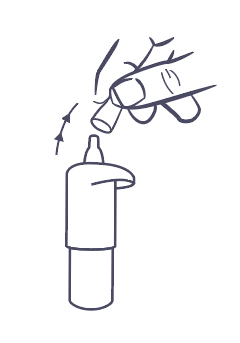

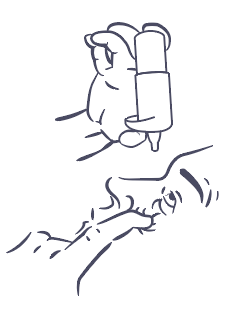
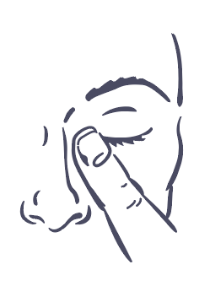
Figure 1.
Figure 2.
Figure 3.
Figure 4.
Use only one bottle at a time.
Use Tipifree Combi for as long as your doctor has recommended.
Using more than the recommended dose of Tipifree Combi
If you have used more Tipifree Combi than the recommended dose, rinse your eyes with warm water. Do not administer more medicine until the next normal dose is due.
Missing a dose of Tipifree Combi
If you miss a dose of Tipifree Combi, continue with the normal dosing schedule. Do not take a double dose to make up for a missed dose. The dose of the medicine administered to the affected eye(s) should not exceed one drop per day.
Stopping the use of Tipifree Combi
If you stop using Tipifree Combi without consulting your doctor, the pressure in your eye will not be controlled, which may lead to loss of vision.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Usually, you can continue using the drops, unless the side effects are severe.
If you are concerned, talk to your doctor or pharmacist. Do not stop using Tipifree Combi without consulting your doctor.
Very common side effects (occurring in more than 1 in 10 patients)
Eyelid symptoms
Eye redness.
Common side effects (occurring in less than 1 in 10 patients)
Eyelid symptoms
Inflammation of the surface of the eye with damage to the surface of the eye, eye pain, blurred vision, abnormal vision, dry eye, eye itching, eye discomfort, objective and subjective symptoms of eye irritation (e.g., burning, stinging).
Uncommon side effects (occurring in less than 1 in 100 patients)
Eyelid symptoms
Inflammation of the surface of the eye, inflammation of the eyelids, swelling of the conjunctiva, increased growth of eyelashes, inflammation of the iris, eye inflammation, sensitivity to light, limited vision, eye fatigue, eye allergy, eye swelling, increased tearing, eyelid redness, changes in eyelid color, darkening of the skin (around the eye).
General symptoms
Allergic reaction to the active substance, dizziness, headache, increased or decreased blood pressure, shortness of breath, excessive hair growth, runny nose, skin rash and itching, slow heart rate.
Rare side effects (occurring in less than 1 in 1,000 patients)
Eyelid symptoms
Damage to the surface of the eye (ulceration), inflammation of the meibomian glands, rupture of blood vessels in the eye, scales on the eyelid margins, abnormal eyelash position, abnormal eyelash growth.
General symptoms
Nervousness, irregular heartbeat, hair loss, voice disorders, breathing difficulties, cough, throat irritation, sore throat, chills, abnormal liver function test results, hives, skin discoloration, increased thirst, fatigue, discomfort in the nose, blue-green urine discoloration, pain in hands and feet.
Side effects with unknown frequency (frequency cannot be estimated from the available data)
Eyelid symptoms
Swelling of the macula, drooping eyelids (causing the feeling of half-closed eyes), eye sinking (eyes appear more deeply set), changes in iris color (colored part of the eye), corneal disorders.
General symptoms
Rash, heart failure, chest pain, stroke, fainting, depression, asthma, increased heart rate, feeling of numbness or tingling, palpitations, swelling of the lower limbs, taste disorders.
Additional information:
Tipifree Combi is a combination of two active substances, travoprost and timolol.
Travoprost and timolol (a beta-adrenergic blocker), like other eye drops, are absorbed into the bloodstream. This may lead to side effects similar to those seen when beta-adrenergic blockers are taken orally or by injection.
The frequency of side effects after administration to the eye is less than after oral or injectable administration.
The following side effects include those observed with the entire group of beta-adrenergic blockers used in ophthalmology or those observed with travoprost alone:
Eyelid symptoms
Inflammation of the eyelids, inflammation of the cornea, detachment of the layer under the retina after filtration surgery, which can cause vision disturbances, reduced corneal sensitivity, corneal ulceration (damage to the front layer of the eyeball), double vision, conjunctival papillae, eye discharge, swelling around the eyes, eyelid itching, eyelid eversion with redness, irritation, and excessive tearing, blurred vision (signs of cataract clouding), swelling of the eye (vascular membrane), rash on the eyelids, seeing halos, reduced eye sensitivity, discoloration inside the eye, pupil dilation, change in eyelash color, change in eyelash texture, abnormal eyelash growth.
General symptoms
Ear and labyrinth disorders: dizziness with a feeling of spinning, ringing in the ears.
Heart and blood vessels: slow heart rate, palpitations, edema (fluid retention), changes in heart rhythm or rate, congestive heart failure (heart disease with shortness of breath and swelling of the ankles and feet due to fluid accumulation), heart rhythm disorder, heart attack, low blood pressure, Raynaud's syndrome, cold hands and feet, reduced blood flow to the brain.
Respiratory system: bronchospasm in the lungs (mainly in patients with pre-existing disease), runny nose or feeling of nasal congestion, sneezing (caused by allergy), breathing difficulties, nosebleeds, dryness of the nasal mucosa.
Nervous system and general disorders: difficulty sleeping (insomnia), nightmares, memory loss, hallucinations, loss of strength and energy, anxiety (excessive emotional distress).
Gastrointestinal system: taste disorders, nausea, indigestion, diarrhea, dry mouth, abdominal pain, vomiting, and constipation.
Allergic reactions: exacerbation of allergic symptoms, generalized allergic reactions including skin edema on areas such as the face and limbs, which can cause respiratory tract obstruction and difficulty swallowing and breathing, localized or generalized rash, itching, severe and sudden life-threatening allergic reaction.
Skin: rash with a white-silver color (resembling psoriasis) or worsening of psoriasis, skin peeling, abnormal hair texture, allergic skin inflammation with itchy rash and redness, hair color change, loss of eyelashes or eyebrows, itching, abnormal hair growth, skin redness.
Musculoskeletal system: worsening of objective and subjective symptoms of myasthenia gravis(muscle disease), abnormal sensations, such as tingling and numbness, muscle weakness/fatigue, muscle pain not caused by physical exercise, joint pain.
Kidney and urinary disorders:difficult and painful urination, urinary incontinence.
Reproductive system:sexual function disorders, decreased libido.
Metabolism:low blood sugar, increased prostate cancer marker levels.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Tipifree Combi
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after EXP. The expiry date refers to the last day of the month stated.
Do not store above 25°C. Store the bottle in the outer carton to protect from light.
Do not freeze.
Shelf life after first opening:
- bottle containing 2.5 ml - 30 days;
- bottle containing 7.5 ml - 90 days. Record the date of opening on the space provided on the carton. After first opening the bottle: Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Tipifree Combi contains
- The active substances are travoprost and timolol. Each ml contains 40 micrograms of travoprost and 5 mg of timolol (as timolol maleate).
- The other ingredients are: boric acid; macrogolglycerol hydroxystearate 40; propylene glycol (E 1520); sodium chloride; mannitol; sodium hydroxide (to adjust pH); purified water.
What Tipifree Combi looks like and contents of the pack
Tipifree Combi is a colorless, clear solution.
The pack consists of an HDPE bottle with a multidose dropper with a pump (PP, HDPE, LDPE), an HDPE cap, and a dosing aid (PP), in a cardboard box.
Each 2.5 ml bottle contains at least 60 drops.
Each 7.5 ml bottle contains at least 180 drops.
Pack sizes:
1 bottle of 2.5 ml
1 bottle of 7.5 ml
Not all pack sizes may be marketed.
Marketing authorization holder
Polpharma S.A.
Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Manufacturer
Jadran - Galenski Laboratorij d.d.
Svilno 20
51000 Rijeka
Croatia
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterJadran-Galenski laboratorij d.d.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tipifree CombiDosage form: Drops, 50 mcg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Pharmaselect International Beteiligungs GmbHPrescription not requiredDosage form: Drops, (0.3 mg + 5 mg)/mlActive substance: timolol, combinationsPrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Genetic S.p.APrescription required
Alternatives to Tipifree Combi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tipifree Combi in Hiszpania
Alternative to Tipifree Combi in Ukraina
Online doctors for Tipifree Combi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tipifree Combi – subject to medical assessment and local rules.














