
How to use Thalidomide Accord
Leaflet accompanying the packaging: patient information
Thalidomide Accord, 50 mg, hard capsules
Thalidomide
WARNING
Thalidomide causes fetal damage and fetal death. It should not be taken if you are pregnant or breastfeeding, or if you plan to become pregnant.
You must follow the doctor's advice on contraception, which will be provided.
You should carefully read the contents of the leaflet before taking the medicine, as it contains important information for the patient.
- You should keep this leaflet, so you can read it again if you need to.
- If you have any doubts, you should consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not give it to others. The medicine may harm another person, even if their symptoms are the same.
- If you experience any side effects, including those not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Thalidomide Accord and what is it used for
- 2. Important information before taking Thalidomide Accord
- 3. How to take Thalidomide Accord
- 4. Possible side effects
- 5. How to store Thalidomide Accord
- 6. Contents of the packaging and other information
1. What is Thalidomide Accord and what is it used for
What is Thalidomide Accord
Thalidomide Accord contains the active substance thalidomide. It belongs to a group of medicines that affect the patient's immune system.
What is Thalidomide Accord used for
Thalidomide Accord is used in combination with two other medicines, melphalan and prednisone, to treat adults with a type of cancer called multiple myeloma. Thalidomide Accord is used in newly diagnosed patients aged 65 years or older who have not received any previous treatment for multiple myeloma, or in patients under 65 years who cannot receive high-dose chemotherapy.
What is multiple myeloma
Multiple myeloma is a type of cancer that affects a specific type of white blood cell called plasma cells. These cells accumulate in the bone marrow and undergo uncontrolled division. This can cause damage to the bones and kidneys. Multiple myeloma cannot be cured, but the symptoms can be significantly reduced or alleviated for a period of time. This is called "remission".
How Thalidomide Accord works
Thalidomide Accord works by stimulating the patient's immune system and directly fighting cancer. The medicine works in several ways:
- by inhibiting the growth of cancer cells,
- by inhibiting the growth of blood vessels in the tumor,
- by stimulating part of the immune system to fight cancer cells.
2. Important information before taking Thalidomide Accord
The doctor will provide specific instructions for taking the medicine, particularly regarding the effects of thalidomide on unborn babies (presented in the "Thalidomide Accord Pregnancy Prevention Program").
When not to take Thalidomide Accord
Warnings and precautions
If any of the following circumstances occur, you should discuss them with your doctor, pharmacist, or nurse before starting treatment with this medicine.
For women taking Thalidomide Accord
Before starting therapy, you should consult your doctor about the possibility of becoming pregnant, even if you think it is unlikely. You can become pregnant even if you do not have menstrual periods due to cancer treatment.
- The doctor will ensure that pregnancy tests are performed before starting therapy, every 4 weeks during treatment, and 4 weeks after the end of treatment,
- You must use one effective method of contraception: for at least 4 weeks before starting therapy, during therapy, and for at least 4 weeks after the end of therapy. The doctor will indicate which contraceptive method should be used.
If you can become pregnant, at each visit for prescription renewal, the doctor will record that the above-mentioned appropriate precautions have been taken and provide you with a confirmation.
For men taking Thalidomide Accord
Thalidomide is present in semen. Therefore, you must not have sexual intercourse without protection, even if you have had a vasectomy.
- You must avoid exposing a pregnant woman or a woman who may become pregnant. You must always use condoms: during treatment and for at least 7 days after the end of treatment,
- You must not donate semen: during treatment and for at least 7 days after the end of treatment.
For all patients
Before starting treatment with Thalidomide Accord, you should tell your doctor if:
- you do not understand the doctor's advice on contraception or do not feel able to follow the recommendations in this regard,
- you have had a heart attack, have ever had blood clots, or if you smoke, have high blood pressure, or high cholesterol levels. During treatment with Thalidomide Accord, there is an increased risk of blood clots in veins and arteries (see also section 4, "Possible side effects"),
- you have had or currently have neuropathy, i.e., nerve damage causing tingling, coordination disorders, or pain in the hands and feet (see also section 4, "Possible side effects"),
- you have had or currently have slow heart rate (which may be a sign of bradycardia),
- you have high blood pressure in the pulmonary arteries (see also section 4, "Possible side effects"),
- you have had a decrease in the number of white blood cells (neutropenia) with accompanying fever and infection,
- you have had a decrease in the number of platelets - you are more prone to bleeding and bruising,
- you have had or currently have liver damage (liver disorders), including abnormal liver enzyme test results,
- you have had or currently have severe skin reactions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, or drug reaction with eosinophilia and systemic symptoms (also known as DRESS). (Description of symptoms, see section 4 "Possible side effects").
- you have had or currently have symptoms of an allergic reaction, such as rash, itching, swelling, dizziness, or difficulty breathing,
- you have had or currently have drowsiness,
- you have had or currently have fever, chills, and severe seizures, which may be accompanied by low blood pressure and disorientation (may be symptoms of severe infections),
- you have had or currently have a viral infection, especially herpes zoster, hepatitis B, or HIV. If in doubt, consult your doctor. Treatment with Thalidomide Accord may cause the reactivation of a virus that you are a carrier of, leading to the recurrence of the infection. The doctor should check if you have been infected with hepatitis B virus in the past,
- you have had or currently have kidney or liver problems (see also section 4, "Possible side effects").
If you experience any of the following at any time during treatment or after its completion, you should immediately inform your doctor or nurse: vision disturbances, loss of vision, or double vision, speech difficulties, weakness in the arms or legs, changes in gait or balance problems, persistent numbness, decreased sensation, or loss of sensation, memory loss, or disorientation. All of these symptoms may indicate a serious and potentially life-threatening brain disease called progressive multifocal leukoencephalopathy (PML). If these symptoms occurred before taking Thalidomide Accord, you should inform your doctor about any changes in these symptoms.
Children and adolescents
Thalidomide Accord should not be used in children and adolescents under 18 years of age.
Thalidomide Accord and other medicines
You should tell your doctor or pharmacist about all medicines you are taking or have recently taken, including those obtained without a prescription, including herbal products.
Taking Thalidomide Accord with food, drink, and alcohol
You should not drink alcohol while taking Thalidomide Accord, as alcohol has a sedative effect, and Thalidomide Accord may enhance this effect.
Pregnancy and breastfeeding
If you are pregnant, breastfeeding, think you may be pregnant, or plan to have a baby, you should consult your doctor or pharmacist before taking this medicine.
- Thalidomide causes severe birth defects and can cause fetal death.
- Even a single capsule of the medicine taken by a pregnant woman can cause serious birth defects in the baby.
- These defects can include: shortening of the arms or legs, deformities of the hands or feet, eye or ear damage, and problems with the internal organs. You must not take Thalidomide Accord if you are pregnant. Additionally, you must not become pregnant while taking Thalidomide Accord. If you can become pregnant, you must use one effective method of contraception (see section 2: "What you need to know before taking Thalidomide Accord").
It is necessary to stop treatment and inform your doctor immediately if:
- You do not have menstrual periods or you think you do not have menstrual periods, or if you experience abnormal menstrual bleeding, or if you suspect you are pregnant.
- You have had heterosexual intercourse without using effective contraception. If you become pregnant during treatment with thalidomide, you should stop treatment immediately and inform your doctor. Men taking Thalidomide Accord who have a female partner who can become pregnant should read the contents of section 2 "What you need to know before taking Thalidomide Accord". If the partner of the patient becomes pregnant during treatment with thalidomide, the patient must immediately inform the treating doctor. Breastfeeding You must not breastfeed while taking Thalidomide Accord. It is not known whether thalidomide passes into breast milk.
Driving and using machines
You should not drive or operate machinery if you experience side effects such as dizziness, fatigue, drowsiness, or vision disturbances.
Thalidomide Accord contains isomalt
If you have been diagnosed with intolerance to some sugars, you should consult your doctor before taking this medicine.
3. How to take Thalidomide Accord
This medicine should always be taken exactly as advised by your doctor or pharmacist. If you are unsure, you should consult your doctor or pharmacist.
How much medicine to take
The recommended dose of Thalidomide Accord for adults under 75 years of age is 200 mg (4 capsules of 50 mg) per day, and for adults over 75 years of age, it is 100 mg (2 capsules of 50 mg) per day. However, the doctor will determine the dose of the medicine suitable for you, monitor the progress of treatment, and may change the dose if necessary.
Taking Thalidomide Accord
- Do not break, open, or chew the capsules. If the powder from a damaged Thalidomide Accord capsule comes into contact with the skin, wash the skin immediately with soap and water.
- Healthcare professionals, caregivers, and family members should wear disposable gloves when handling the blister or capsule. The gloves should then be carefully removed to avoid skin exposure, placed in a sealed plastic bag, and disposed of according to local regulations. Then, wash your hands thoroughly with soap and water. Pregnant women or those who suspect they are pregnant should not touch the blister or capsule.
- Take the medicine orally.
- Swallow the capsules whole with a glass of water.
- Do not crush or chew the medicine.
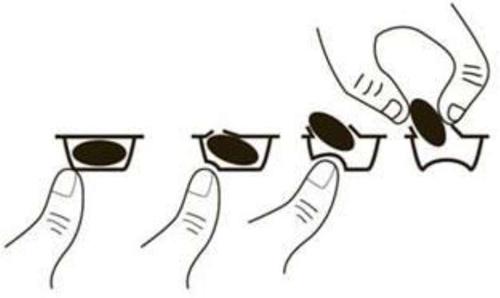
- Take the capsules as a single dose before bedtime. This will reduce the likelihood of you feeling drowsy during the day. To remove a capsule from the blister, press the capsule from one side only and push it through the foil. Do not press the center of the capsule, as this may damage it.
Overdose of Thalidomide Accord
If you have taken more Thalidomide Accord than prescribed, you should consult your doctor or go to the hospital immediately. If possible, take the packaging and this leaflet with you.
Missing a dose of Thalidomide Accord
If you forget to take a dose of Thalidomide Accord at the usual time, and
- it has been less than 12 hours since then: you should take the capsules immediately.
- it has been more than 12 hours since then: you should not take the capsules. You should take the next dose of the medicine at the usual time the next day. If you have any further doubts about taking this medicine, you should consult your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Thalidomide Accord can cause side effects, although not everybody gets them.
You must stop taking Thalidomide Accord and contact your doctor immediately if you notice any of the following serious side effects - you may need urgent medical treatment:
- Severe and serious skin reactions. Adverse skin reactions can occur in the form of rashes with or without blisters. They may include:
- skin irritation, sores, or swelling in the mouth, throat, eyes, nose, and genital area, swelling, and fever, as well as flu-like symptoms. These symptoms may be a sign of rare and serious skin reactions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, or DRESS.
- Allergic reactions, such as localized or generalized itching rash, angioedema, or anaphylactic reaction (severe forms of allergic reactions, which can manifest as hives, rash, swelling of the eyes, lips, or face, difficulty breathing, or itching).
You should inform your doctor immediately if you experience any of the following serious side effects:
- Numbness, tingling, coordination disorders, or pain in the hands and feet.
This symptom may be a sign of nerve damage (peripheral neuropathy), which is a very common side effect. It can be very severe, causing pain and leading to disability. If you experience such symptoms, you should immediately contact your doctor, who may reduce the dose of the medicine or stop treatment. This side effect usually occurs after a longer period of treatment, but it can also occur earlier. It can also occur after the end of treatment. The symptom may not disappear or may disappear slowly.
Sudden chest pain or difficulty breathing
This symptom may be a sign of a blood clot in the arteries leading to the lungs (pulmonary embolism), which is a common side effect. This symptom can occur during treatment or after its completion.
Pain or swelling in the legs, especially in the lower part or calves
This symptom may be a sign of blood clots in the veins of the legs (deep vein thrombosis), which is a common side effect. This symptom can occur during treatment or after its completion.
Chest pain radiating to the arms, neck, jaw, back, or stomach, feeling of choking and shortness of breath, malaise, or vomiting.
These may be symptoms of a heart attack (which can occur due to blood clots in the blood vessels of the heart).
Transient vision or speech disturbances
These may be symptoms of a stroke (which can occur due to blood clots in the blood vessels of the brain).
Fever, chills, sore throat, cough, mouth sores, or other signs of infection.
Bleeding or bruising without injury.
Other side effects include:
Note that in a small number of patients with multiple myeloma, other types of cancer, particularly hematological malignancies, may develop, and it is possible that the risk may increase with the use of Thalidomide Accord. Therefore, the doctor should carefully weigh the benefits and risks of prescribing Thalidomide Accord to the patient.
Very common (may affect more than 1 in 10 people)
- Constipation
- Dizziness
- Drowsiness, fatigue
- Tremors
- Weak or abnormal sensation
- Swelling of the hands and feet
- Decreased number of blood cells. This may mean a greater susceptibility to infections. During treatment with Thalidomide Accord, the doctor may monitor blood test results.
Common (may affect up to 1 in 10 people)
- Nausea, vomiting, feeling of dryness in the mouth
- Rash, dry skin
- Decreased number of white blood cells (neutropenia) with accompanying fever and infection.
- Simultaneous decrease in the number of red and white blood cells and platelets (pancytopenia)
- Weakness, feeling of weakness and instability, lack of energy or strength, low blood pressure,
- Fever, general malaise
- Seizures
- Dizziness when standing up and moving normally
- Vision disturbances
- Lung inflammation, lung disease
- Slow heart rate, heart failure
- Depression, disorientation, mood changes, anxiety
- Hearing loss or deafness
- Kidney disease (kidney failure)
Uncommon (may affect up to 1 in 100 people)
- Inflammation and swelling of the airways in the lungs (bronchitis)
- Inflammation of the cells lining the stomach
- Perforation (puncture) of the large intestine, which can lead to infection
- Intestinal obstruction
- Low blood pressure when standing up, which can lead to fainting
- Irregular heartbeat (heart block or atrial fibrillation), feeling of weakness, and fainting
Unknown frequency (frequency cannot be estimated from the available data)
- Insufficient thyroid function (hypothyroidism),
- Sexual function disorders, such as impotence,
- Severe blood infection (sepsis) with fever, chills, and severe shivering, with possible complications - low blood pressure and confusion (septic shock),
- Syndrome of tumor breakdown - metabolic complications that occur during cancer treatment, and sometimes even without treatment. These complications are caused by the breakdown products of dying cancer cells and may include: changes in blood chemistry; high levels of potassium, phosphorus, uric acid, and low levels of calcium, leading to kidney function disorders, heart rhythm disturbances, seizures, and in some cases, death,
- Liver damage (liver disorders), including abnormal liver enzyme test results,
- Bleeding from the stomach or intestines (gastrointestinal bleeding),
- Worsening of Parkinson's disease symptoms (such as tremors, depression, or disorientation),
- Pain in the upper abdomen and (or) back, which can be severe and last for several days, with possible accompanying nausea, vomiting, fever, and rapid heartbeat - these symptoms may be due to pancreatitis,
- Increased blood pressure in the pulmonary arteries, which can lead to symptoms such as shortness of breath, fatigue, dizziness, chest pain, rapid heartbeat, swelling of the legs or ankles (pulmonary hypertension),
- Viral infections, including herpes zoster and hepatitis B virus reactivation (which can cause yellowing of the skin and eyes, dark urine, abdominal pain on the right side, fever, nausea, and vomiting),
- Brain disease with symptoms such as vision disturbances, headache, seizures, and disorientation, with or without high blood pressure (posterior reversible encephalopathy syndrome or PRES),
- Skin disease caused by inflammation of small blood vessels, with accompanying joint pain and fever (leukocytoclastic vasculitis).
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Jerozolimskie Avenue 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder.
5. How to store Thalidomide Accord
This medicine should be stored out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging and on the blister after "Expiry date" / "EXP". The expiry date refers to the last day of the month stated.
Do not use this medicine if you notice damage or signs of tampering with the packaging.
Do not store above 30°C.
After completing treatment, you must return all unused capsules to your pharmacist or doctor. This will prevent the misuse of the medicine.
6. Contents of the packaging and other information
What Thalidomide Accord contains
- The active substance of the medicine is thalidomide. Each capsule contains 50 mg of thalidomide.
- Other ingredients are: The capsule contains isomalt (E 953), croscarmellose sodium, sodium stearyl fumarate. The capsule shell contains gelatin and titanium dioxide (E 171).
What Thalidomide Accord looks like and contents of the pack
Thalidomide Accord is a white, opaque, hard capsule, size 4.
The capsule filling is a white powder.
PVC/PCTFE/Aluminum blister pack containing 14 capsules.
Package sizes: 28 capsules (2 blisters) in a wallet card carton.
Single-dose PVC/PCTFE/Aluminum blister pack containing 7 capsules.
Package sizes: 28 capsules (4 blisters) in a carton.
Marketing authorization holder
Accord Healthcare Polska Sp. z o.o., Taśmowa 7, 02-677 Warsaw
Manufacturer
Ardena Pamplona, S.L., Polígono Mocholí, Noáin N 1, 31110 Navarra, Spain
Medichem S.A., Narcís Monturiol, 41 A, Sant Joan Despí, 08970 Barcelona, Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Date of last revision of the leaflet: March 2022
| Country name | Marketing authorization holder |
| Cyprus | Thalidomide Accord 50mg σκληρά καψάκια |
| Denmark | Thalidomide Accord |
| France | Thalidomide Accord 50mg gélule |
| Spain | Talidomida Accord 50mg cápsulas duras |
| Malta | Thalidomide Accord 50 mg hard capsules |
| Poland | Thalidomide Accord |
| Portugal | Thalidomide Accord 50mg cápsulas |
| Romania | Thalidomide Accord 50mg capsule |
| Slovenia | Talidomid Accord 50mg trde kapsule |
| Hungary | Thalidomide Accord 50mg kemény kapszula |
| Italy | Talidomide Accord |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterArdena Pamplona S.L. Medichem S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Thalidomide AccordDosage form: Capsules, 120 mgActive substance: dimethyl fumarateManufacturer: Pharmathen International S.A. Pharmathen S.A.Prescription requiredDosage form: Capsules, 240 mgActive substance: dimethyl fumarateManufacturer: Pharmathen International S.A. Pharmathen S.A.Prescription requiredDosage form: Capsules, 120 mgActive substance: dimethyl fumaratePrescription required
Alternatives to Thalidomide Accord in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Thalidomide Accord in Spain
Alternative to Thalidomide Accord in Ukraine
Online doctors for Thalidomide Accord
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Thalidomide Accord – subject to medical assessment and local rules.






