

Tetraspan 60 mg/ml Hes roztvur do infuzii

Ask a doctor about a prescription for Tetraspan 60 mg/ml Hes roztvur do infuzii

How to use Tetraspan 60 mg/ml Hes roztvur do infuzii
PATIENT INFORMATION LEAFLET: USER INFORMATION
Tetraspan, 60 mg/ml, solution for infusion
Warning
Do not use in case of sepsis (severe generalized infection), renal dysfunction, or in critically ill patients.
Situations in which this product should never be used are listed in section 2.
This medicinal product will be subject to additional monitoring. This will allow for quick identification of new safety information. The user of the medicinal product can also help by reporting any adverse reactions that occur after using the medicinal product. To find out how to report adverse reactions, see section 4.
Report adverse reactions – see section 4.
You should read the contents of the leaflet before using the medicinal product, as it contains important information for the patient.
- You should keep this leaflet, so you can read it again if you need to.
- You should consult a doctor or pharmacist if you have any further questions.
- This medicinal product has been prescribed specifically for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any adverse reactions, including any possible adverse reactions not listed in the leaflet, you should tell your doctor or pharmacist.
Table of contents of the leaflet:
- 1. What is Tetraspan 60 mg/ml and what is it used for
- 2. Important information before using Tetraspan 60 mg/ml
- 3. How to use Tetraspan 60 mg/ml
- 4. Possible adverse reactions
- 5. How to store Tetraspan 60 mg/ml
- 6. Contents of the pack and other information
1. What is Tetraspan 60 mg/ml and what is it used for
Tetraspan 60 mg/ml is a solution for infusion administered through a vein.
Tetraspan 60 mg/ml is a plasma substitute used to restore blood volume due to its loss, when the use of crystalloid products is not sufficient.
2. Important information before using Tetraspan 60 mg/ml
When not to use Tetraspan 60 mg/ml:
- in patients allergic to any of the active substances or any of the other ingredients of this medicinal product (listed in section 6);
- in patients with severe generalized infection (sepsis);
- in patients with burns;
- in patients with renal failure or undergoing dialysis;
- in patients with severe liver disease;
- in patients with intracranial hemorrhage (intracranial or cerebral hemorrhage);
- in critically ill patients (e.g., those in the Intensive Care Unit);
- in patients with excessive fluid in the body and who have been informed that they have a state of overhydration;
- in patients with fluid in the lungs (pulmonary edema);
- in dehydrated patients;
- in patients with a significant increase in potassium, sodium, or chloride levels in the blood;
- in patients with severe liver dysfunction;
- in patients with severe heart failure;
- in patients with severe coagulation disorders;
- in patients after organ transplantation.
Warnings and precautions
Before starting treatment with Tetraspan 60 mg/ml, you should tell your doctor if you have:
- liver dysfunction;
- heart and circulation disorders;
- coagulation disorders;
- renal dysfunction.
Due to the risk of an allergic reaction(anaphylactic/anaphylactoid), your doctor will closely monitor your condition to observe early signs of an allergic reaction after administration of this medicinal product.
Surgical procedures and injuries:
Your doctor will consider whether this medicinal product is suitable for you.
Your doctor will carefully determine the dose of Tetraspan 60 mg/ml to avoid fluid overload, especially in patients with lung, heart, or circulation problems. If you have a blood infection or renal dysfunction, or if there is a risk of renal dysfunction, your doctor will closely monitor blood circulation (hemodynamic functions).
Nursing staff will also take measures to monitor fluid balance, electrolyte levels, and renal function. If necessary, you may receive additional mineral salts.
Additionally, you will be ensured an adequate supply of fluids.
Tetraspan 60 mg/ml is contraindicated in patients with renal dysfunction or renal damage requiring dialysis.
If renal dysfunction occurs during treatment:
If your doctor observes the first signs of renal dysfunction, they will stop administering this medicinal product. Additionally, there may be a need to monitor renal function for up to 90 days.
If Tetraspan 60 mg/ml is administered repeatedly, your doctor will monitor blood coagulation, bleeding time, and other parameters. If coagulation is impaired, your doctor will discontinue administration of this medicinal product.
The use of this medicinal product is not recommended in patients undergoing open-heart surgery, connected to heart-lung machines that support blood pumping during the procedure.
Elderly patients
Your doctor will closely monitor your condition during treatment and may adjust the dose, considering that elderly patients are more prone to kidney and heart problems.
Other medicinal products and Tetraspan 60 mg/ml
You should tell your doctor or pharmacist about all medicinal products you are currently taking or have recently taken, as well as any medicinal products you plan to take.
Your doctor will exercise special caution when prescribing this medicinal product if you are receiving/taking:
- aminoglycosides (a group of antibiotics),
- medicinal products that can cause potassium or sodium retention,
- medicinal products used to treat heart failure (e.g., digitalis preparations).
Tetraspan 60 mg/ml may enhance the effects of these medicinal products.
Pregnancy and breastfeeding
In pregnancy and during breastfeeding, or if you think you may be pregnant, or if you plan to become pregnant, you should consult your doctor or pharmacist before using this medicinal product.
Pregnancy
The occurrence of an allergic reaction to the medicinal product, due to the use of hydroxyethyl starch, may have a harmful effect on the unborn child.
You will receive this medicinal product only if your doctor considers that the potential benefits outweigh the possible risk to the unborn child, especially if you are in the first trimester of pregnancy.
Breastfeeding
It is not known whether hydroxyethyl starch passes into breast milk. Therefore, your doctor will administer this solution only if they consider it necessary and will decide whether to temporarily stop breastfeeding.
Driving and using machines
Tetraspan 60 mg/ml does not affect the ability to drive and use machines.
3. How to use Tetraspan 60 mg/ml
Tetraspan 60 mg/ml is administered intravenously through a drip (intravenous infusion).
Dosage
Your doctor will adjust the dose to your needs.
Your doctor will use the smallest effective dose and limit the infusion time of Tetraspan 60 mg/ml to a maximum of 24 hours.
Adults
The maximum daily dose is 30 ml/kg body weight (which corresponds to 1.8 g of hydroxyethyl starch per kg body weight).
Children
There are limited data on the use of this medicinal product in children. It is not recommended to use this medicinal product in children.
Elderly patients and patients in a special condition
In the case of elderly patients, patients with renal dysfunction, lung, heart, or circulation problems, the dose of the medicinal product will be adjusted to the individual patient's condition.
Using a higher dose of Tetraspan 60 mg/ml than recommended
If you have received a higher dose of Tetraspan 60 mg/ml than recommended, you may experience circulatory overload, which can affect heart and lung function.
If this occurs, your doctor will immediately stop the infusion of Tetraspan 60 mg/ml and initiate necessary treatment.
If you have any further questions about the use of this medicinal product, you should consult your doctor or pharmacist.
4. Possible adverse reactions
Like all medicinal products, this medicinal product can cause adverse reactions, although not everybody gets them.
The most commonly observed adverse reactions are directly related to the therapeutic effect of starch solutions and the administered doses, i.e., dilution of the patient's blood and the blood components responsible for its coagulation. In addition, severe allergic reactions have been observed.
The following adverse reactions may be serious. If you experience any of the following adverse reactions, you should stop using the medicinal product and consult your doctor immediately.
Very common(may affect more than 1 in 10 patients):
decreased red blood cell count and decreased protein levels in the blood due to dilution.
Common, depending on the dose(may affect more than 1 in 100 patients):
dilution of blood coagulation factors (blood components responsible for blood coagulation). This may lead to bleeding complications.
Rare(may affect up to 1 in 1,000 patients):
Allergic reactions may occur regardless of the dose. They may be severe and lead to shock. If an allergic reaction occurs, in particular an anaphylactic reaction (including facial swelling, tongue or throat swelling, difficulty swallowing, hives, and difficulty breathing), your doctor will immediately stop the infusion of Tetraspan 60 mg/ml and take basic measures.
It is not possible to predict which patients may experience an allergic reaction, or the course or severity of such an allergic reaction.
Frequency not known (cannot be estimated from the available data):
- kidney damage;
- liver damage.
Other adverse reactions
Very common(may affect more than 1 in 10 patients):
Hydroxyethyl starch infusion causes an increase in alpha-amylase enzyme levels in serum, which may be misinterpreted as evidence of pancreatic disorder.
Uncommon(may affect up to 1 in 100 patients):
In patients receiving multiple infusions of Tetraspan 60 mg/ml over several days, itching may occur after treatment, which may appear even several weeks after the end of treatment. Itching may persist for several months.
Reporting adverse reactions
If you experience any adverse reactions, including any possible adverse reactions not listed in the leaflet, you should tell your doctor or nurse. Adverse reactions can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products {current address, phone number, and fax of the aforementioned Department} e-mail: [email protected].
Reporting adverse reactions can help gather more information on the safety of the medicinal product.
5. How to store Tetraspan 60 mg/ml
The medicinal product should be stored out of sight and reach of children.
Do not use this medicinal product after the expiry date stated on the label. The expiry date refers to the last day of the given month.
For single use only. After use, the container and any unused solution should be disposed of.
Do not freeze.
Do not use the medicinal product if the solution is not clear, colorless, or if the container or its closure is damaged.
6. Contents of the pack and other information
What Tetraspan 60 mg/ml contains
- Active substance content per 1,000 ml of solution:
Hydroxyethyl starch (HES)
60.0 g
(Substitution degree:
0.42)
(Average molecular weight:
130,000 Da)
Sodium chloride
6.25 g
Potassium chloride
0.30 g
Calcium chloride dihydrate
0.37 g
Magnesium chloride hexahydrate
0.20 g
Sodium acetate trihydrate
3.27 g
Lactic acid
0.67 g
- Other ingredients are: sodium hydroxide (for pH adjustment) Water for injections.
Electrolyte concentrations:
Sodium
140.0 mmol/l
Potassium
4.0 mmol/l
Calcium
2.5 mmol/l
Magnesium
1.0 mmol/l
Chloride
118.0 mmol/l
Acetate
24.0 mmol/l
Lactate
5.0 mmol/l
pH:
5.6-6.4
Theoretical osmolality:
296 mOsm/l
Acidity (titrated to pH 7.4):
<2.0 mmol/l
What Tetraspan 60 mg/ml looks like and what the pack contains
Clear, colorless aqueous solution.
Tetraspan 60 mg/ml is available in the following pack sizes:
- Polyethylene bottle (Ecoflac plus) available in the following packs: 10 x 500 ml
- Plastic bag (Ecobag) with a rubber stopper and an outer polypropylene bag, available in the following packs: 20 x 250 ml 20 x 500 ml
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
- B. Braun Melsungen AG Carl-Braun-Strasse 1 34212 Melsungen Germany
Postal address:
34209 Melsungen, Germany
Phone:
+49 5661 71 0
Fax:
+49 5661 71 4567
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria
Tetraspan 6% Infusionslösung
Belgium
Tetraspan 6% oplossing voor infusie
Czech Republic
Tetraspan 6%
Denmark
Tetraspan 60 mg/ml infusionsvæske
Estonia
Tetraspan 60 mg/ml infusioonilahus
Finland
Tetraspan 60 mg/ml infuusioneste, liuos
Germany
Tetraspan 6% Infusionslösung
Greece
Tetraspan διάλυμα για έγχυση 6%
Hungary
Tetraspan 60 mg/ml oldatos infúzió
Ireland
EquiHes 60 mg/ml Solution for Infusion
Iceland
Tetraspan 60 mg/ml innrennslislyf, lausn
Italy
Tetraspan 60 mg/ml soluzione per infusione
Latvia
Tetraspan 60 mg/ml šķīdum infūzijām
Lithuania
Tetraspan 60 mg/ml infuzinis tirpalas
Luxembourg
Tetraspan 6% Infusionslösung
Norway
Tetraspan 60 mg/ml infusjonsvæske, oppløsning
Poland
Tetraspan 60 mg/ml roztwór do infuzji
Portugal
Tetraspan 6% solução para perfusão
Slovakia
Tetraspan 6%
Slovenia
Tetraspan 60 mg/ml raztopina za infundiranje
Spain
Isohes 6% Solución para perfusión
Sweden
Tetraspan 60 mg/ml infusionsvätska, lösning
Netherlands
Tetraspan 6% g/v, oplossing voor infusie 60g/l
United Kingdom
Tetraspan 6% Solution for Infusion
Date of last revision of the leaflet: 26.10.2018
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
The following information is intended for healthcare professionals only:
The use of hydroxyethyl starch-containing medicinal products should be limited to the initial period of restoring intravascular fluid volume, with a maximum administration period of 24 hours.
The initial 10-20 ml should be administered slowly, with close monitoring of the patient to quickly detect any anaphylactic/anaphylactoid reaction.
The smallest effective dose should be used. Treatment should be carried out with continuous monitoring of hemodynamics, so that the infusion is stopped as soon as the appropriate hemodynamic parameters are achieved. The maximum recommended daily dose should not be exceeded.
The product should be used immediately after opening the direct packaging. Any unused solution should be discarded.
For single use only. Partially used containers should not be reconnected.
Use only if the solution is clear, colorless, and the container is not damaged.
Incompatibilities
Do not mix the medicinal product with other medicinal products, as compatibility studies have not been performed.
Tetraspan 60 mg/ml is iso-oncotic:
Tetraspan 60 mg/ml is an iso-oncotic solution, meaning that the increase in intravascular volume corresponds to the volume of the administered solution.
Instructions for performing a pressure infusion of Tetraspan:
Plastic containers Ecoflac plus and Ecobag:
If the solution is to be administered very quickly under pressure, before starting the procedure, you should completely remove the air from both the plastic containers and the infusion set to prevent the possibility of air embolism during fluid administration. Pressure infusion should be performed using a pressure cuff.
Ecobag:
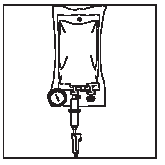
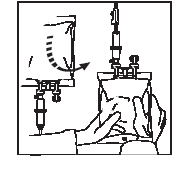
1 2
Connect the infusion set.
Place the container in a vertical position.
Open the roller clamp, squeeze out the air from the container, and fill the drip chamber with fluid halfway.
Rotate the container 180° and fill the infusion tube to remove air bubbles.
Close the roller clamp.
Place the Ecobag container in a pressure cuff.
Increase the pressure.
Open the roller clamp and start the infusion.
Ecoflac plus:
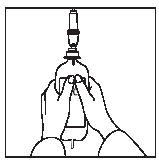
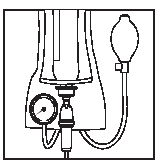
1 2
Connect the infusion set.
Place the container in a vertical position.
Open the roller clamp, squeeze out the air from the container, and fill the drip chamber with fluid halfway.
Rotate the container 180° and fill the infusion tube to remove air bubbles.
Close the roller clamp.
Place the Ecoflac plus container in a pressure cuff.
Increase the pressure.
Open the roller clamp and start the infusion.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterB. Braun Melsungen AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tetraspan 60 mg/ml Hes roztvur do infuziiDosage form: Solution, -Active substance: hydroxyethylstarchPrescription requiredDosage form: Solution, 10% + 0.9%Active substance: hydroxyethylstarchManufacturer: Fresenius Kabi Deutschland GmbHPrescription requiredDosage form: Solution, (60 mg + 9 mg)/mlActive substance: hydroxyethylstarchPrescription not required
Alternatives to Tetraspan 60 mg/ml Hes roztvur do infuzii in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tetraspan 60 mg/ml Hes roztvur do infuzii in Ukraina
Alternative to Tetraspan 60 mg/ml Hes roztvur do infuzii in Hiszpania
Online doctors for Tetraspan 60 mg/ml Hes roztvur do infuzii
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tetraspan 60 mg/ml Hes roztvur do infuzii – subject to medical assessment and local rules.














