

Testavan

Ask a doctor about a prescription for Testavan

How to use Testavan
PATIENT INFORMATION LEAFLET
Leaflet accompanying the packaging: information for the patient
Testavan, 20 mg/g, transdermal gel
Testosterone
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Testavan and what is it used for
- 2. Important information before using Testavan
- 3. How to use Testavan
- 4. Possible side effects
- 5. How to store Testavan
- 6. Contents of the pack and other information
1. What is Testavan and what is it used for
What is Testavan
Testavan is a clear gel that contains testosterone, a male hormone produced naturally in the body of a man.
Testavan is used to supplement the level of testosterone in adult men who do not produce sufficient amounts of natural testosterone, a condition known as hypogonadism. This medicine helps increase testosterone levels to a normal level.
How testosterone works
Testosterone is naturally produced in the body by the testes.
- It helps produce sperm and develop and maintain male characteristics, such as a deep voice and body hair.
- It is necessary for normal sexual function and sex drive.
- It also helps maintain muscle mass and strength.
What Testavan is used for
Testavan is used in men as testosterone replacement therapy for the treatment of various diseases that result from a deficiency of testosterone (male hypogonadism). This should be confirmed by two separate measurements of testosterone levels and the presence of clinical symptoms, such as:
impotence,
infertility,
decreased sex drive,
fatigue,
depressive mood,
bone mass loss due to low hormone levels,
partial loss of secondary sex characteristics, such as changes in voice, changes in fat distribution
and partial loss of facial and body hair.
2. Important information before using Testavan
Who can use Testavan
- Only men can use Testavan.
- Men under 18 years of age should not use this medicine.
- Women, regardless of age, should not use this medicine.
- Women (especially those who are pregnant or breastfeeding) and children should not come into contact with the Testavan gel or the skin areas where the Testavan medicine has been applied.
When not to use Testavan
if the patient is allergic to testosterone or any of the other ingredients of this medicine (listed in section 6),
if the patient has been diagnosed with or is suspected of having prostate cancer,
if the patient has been diagnosed with or is suspected of having breast cancer (rare in men).
Warnings and precautions
Testosterone treatment may accelerate the development of existing prostate cancer.
Before starting treatment with Testavan, the doctor will perform all necessary tests and then periodically check blood parameters and examine the prostate.
Before starting treatment with Testavan, the patient should discuss with their doctor if they have or have had any of the following conditions:
the patient has difficulty urinating due to an enlarged prostate,
the patient has bone cancer; the doctor will check the patient's blood calcium levels,
the patient has high blood pressure or is taking medications for high blood pressure, as Testavan may cause an increase in blood pressure,
the patient has severe heart, liver, or kidney disease, as the use of Testavan may cause serious complications such as fluid retention in the body with sometimes (congestive) heart failure (overload of the heart with fluid),
the patient has coronary heart disease (which affects blood flow to the heart),
blood clotting disorders
- thrombophilia (abnormalities in blood clotting, increasing the risk of thrombosis - the formation of blood clots in blood vessels)
- factors that increase the risk of blood clots in the veins: previous blood clots in the veins; smoking; obesity; cancer; immobilization; a family history of blood clots in the leg, lungs, or other organs at a young age (e.g. under 50 years); the patient's advanced age.
- How to recognize the occurrence of a blood clot: painful swelling of one leg or sudden change in skin color, e.g. paleness, redness, or bruising of the skin, sudden shortness of breath, sudden unexplained cough, which may be accompanied by coughing up blood; sudden chest pain, severe dizziness or fainting, severe abdominal pain, sudden loss of vision. If any of these symptoms occur, seek medical help immediately. if the patient has epilepsy, the patient has migraines, the patient has breathing problems during sleep - these are more likely to occur in patients who are overweight or have chronic lung disease. If the patient has (or may have) any of the above conditions, they should consult their doctor or pharmacist before using Testavan - the use of this medicine may exacerbate these conditions.
If severe skin reactions occur at the site of application of Testavan, the patient should consult their doctor. It may be necessary to discontinue treatment with Testavan.
The following blood tests should be prescribed by the doctor before and during treatment:
testosterone levels in the blood, full blood count.
During and immediately after daily application of Testavan, do not use moisturizing products or sun protection products at the site of application.
How to prevent the transfer of Testavan to another person
Testavan can be transferred to other people through direct skin contact. This can cause an increase in testosterone levels in these people and can be dangerous.
This can happen after a single contact, but it can also develop gradually after contact with small amounts over time.
- This is especially important for women and children - they usually have low levels of testosterone in their bodies.
- Pregnant women must not come into contact with Testavan. If the patient's partner is pregnant, the patient must be careful and protect her from any contact with the medicine and the site of application.
To avoid transferring the gel from the patient's skin to another person, the patient should:
use the applicator and not their fingers to apply the medicine,
wash their hands with soap and water immediately after getting Testavan on their skin,
cover the application site with clothing as soon as the gel has dried,
take a bath or shower, or wear clothing that covers the application site (e.g. a T-shirt) before coming into direct skin contact with another person.
To increase the safety of their partner, the patient should wash the application site with soap and water before sexual intercourse, for example, during a shower, or, if this is not possible, wear a T-shirt that covers the application site during contact.
What to do if another person is exposed to Testavan
If another person touches the gel or comes into direct skin contact with the site of application on the patient's skin, that person should wash the skin in the area of contact with soap and water as soon as possible.
The longer the gel remains in contact with the skin before it is washed, the greater the risk that the person will absorb some testosterone.
If Testavan has been transferred to another person, attention should be paid to any changes in the body or behavior of people around the patient. If any of the following symptoms occur in someone around the patient, that person should see their doctor:
- acne
- deepening of the voice
- increased hair growth on the face or body
- changes in menstruation
- premature sexual development, enlargement of sexual organs, or changes in sexual behavior in children
Testavan and other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and any medicines you plan to take.
Testavan may affect the way they work and you may need to adjust the dose.
In particular, tell your doctor or pharmacist if you are taking:
medicines that reduce blood clotting (anticoagulants) - Testavan may enhance the effect of these medicines,
corticosteroids or any other medicines that may increase the production of these hormones. Corticosteroids and Testavan may cause fluid retention in the body,
insulin to maintain normal blood sugar levels (in diabetes); during treatment with Testavan, it may be necessary to reduce the dose of insulin.
If any of the above circumstances apply (or may apply) to you, consult your doctor or pharmacist before using Testavan.
Pregnancy, breastfeeding, and fertility
Testavan is not indicated for use in pregnant or breastfeeding women.
If the patient's partner is pregnant or becomes pregnant, the patient must follow the instructions above under the heading “How to prevent the transfer of Testavan to another person”.
Pregnant women must avoid anyskin contact with the areas of application of Testavan in men. This medicine may harm the unborn child. In case of contact, wash the skin with soap and water as soon as possible.
Breastfeeding women must avoid anyskin contact with the areas of application of Testavan in men.
Testavan may reversibly suppress sperm production.
Testavan contains propylene glycol
This medicine contains propylene glycol, which may cause skin irritation.
Testavan contains ethanol
This medicine contains 538.70 mg of alcohol (ethanol) in each dose containing 1.15 g of gel, which is equivalent to 468.40 mg/g (46.84% w/w). It may cause a burning sensation on damaged skin.
3. How to use Testavan
This medicine should always be used exactly as directed by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist.
How much medicine to use
- The recommended starting dose of Testavan is 23 mg of testosterone (1 pump actuation) applied once daily at approximately the same time, preferably in the morning. Some patients may require a higher dose.
- The maximum recommended dose is 69 mg of testosterone per day (3 pump actuations).
Your doctor will determine the appropriate dose of Testavan for you, regularly monitoring:
- testosterone levels in your blood
- your response to the medicine. If you think the effect of the medicine is too strong or too weak, talk to your doctor.
Using this medicine
It is important that you read and follow the instructions for the proper use of Testavan.
Testavan should be applied to the skin using the applicator. This is called "transdermal administration"
- the medicine passes through the skin into the body.
The medicine should be applied only to clean, dry, and healthy skin on the upper arm and shoulder.
Never apply Testavan to:
- the penis and testes,
- cracked or damaged skin,
- open wounds, cuts, or skin irritations.
Application requires:
- Testavan application kit See Figure A.
- A box of single-use wipes.
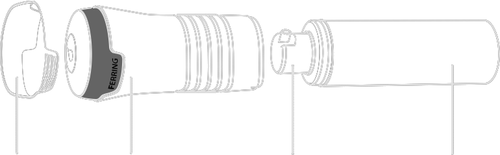
Cap Applicator Pump head Pump actuator
Figure A
Note: Bulk packaging contains containers with a nozzle on top of each pump head.
Preparing the pump actuator for gel - only the first time
Before using a newpump actuator of Testavan, it must be prepared for use. This is called "priming" the pump.
- Detach the applicator from the pump actuator in the case of a single pack or remove the cap from the pump head in the case of a bulk pack. See Figure B.

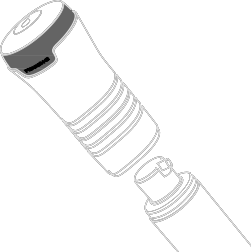
Figure B
- Prime the pump by pressing the pump head all the way down over a wipe until gel appears. See Figure C.
Figure C
- Do not use any gel dispensed during priming, as it may not be the correct dose.
- Discard the used wipe with caution to prevent transfer of the gel to other people, including children and pets.
- The Testavan application kit is now ready for use.
To administer the daily dose, follow steps 1-4
Step 1: Preparing to apply the Testavan gel
- Choose a clean, dry, undamaged area of skin on the upper arm and shoulder that can be covered with a short-sleeved T-shirt. See Figure D.
- Apply Testavan gel onlyto the upper arm and shoulder.
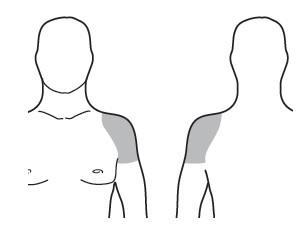
Figure D
- Detach the applicator from the pump actuator. See Figure E.

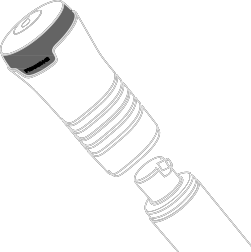
Figure E
- Remove the applicator cap. See Figure F.
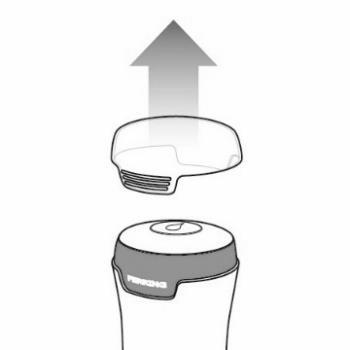
Figure F
Step 2: Applying the Testavan gel
- Hold the pump actuator with the nozzle pointing towards the applicator surface.
- Press the pump head onceall the way down. See Figure G.
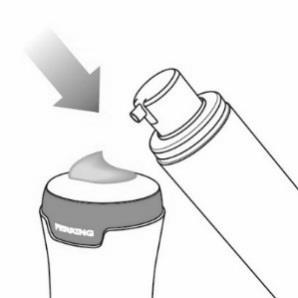
Figure G
- Use the applicator to spread the gel evenly on the skin of the upper arm and shoulder, making sure the gel does not get on the skin of the hands. See Figure H.
- Wipe up any spills with a wipe. Discard the used wipe with caution to prevent transfer of the gel to other people, including children and pets.
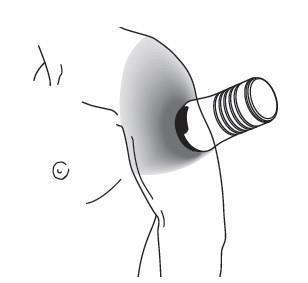
Figure H
Find the doseprescribed by your doctor in the table below.
| Dose | Administration method |
| 23 mg (1 pump actuation) | Apply the gel released after one pump actuation, as shown in Step 2. - do this once. |
| 46 mg (2 pump actuations) | Apply the gel released after one pump actuation, as shown in Step 2. Repeat to apply the gel released after one (second) pump actuation - apply the gel to the upper arm and shoulder on the opposite side. |
| 69 mg (3 pump actuations) | Apply the gel released after one pump actuation, as shown in Step 2. Repeat to apply the gel released after one (second) pump actuation - apply the gel to the upper arm and shoulder on the opposite side. Repeat again to apply the gel released after one (third) pump actuation - apply the gel again to the upper arm and shoulder on the first side. |
Step 3: Cleaning the Testavan applicator
- After use, clean the applicator with a wipe. See Figure I.
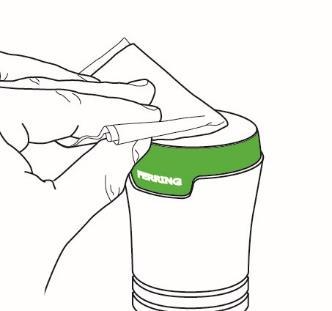
Figure I
- Discard the used wipe with caution. Be careful to prevent accidental transfer of the gel to other people, including children and pets.
- REMEMBERto put the cap back on the applicator and reattach the applicator to the pump actuator . See Figure J.

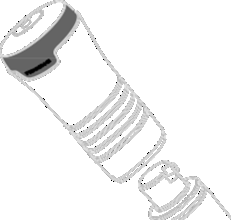
Figure J
- Store the medicine with caution and out of reach of children.
Step 4: After applying Testavan
Wash your hands immediately with soap and water if the gel came into contact with the skin of your hands.
- Testavan gel is flammable before it dries. Wait for the gel to dry before smoking or going near an open flame.
- Wait for the gel to dry before putting on clothes.
- Wear clothes that cover the application site at all times to prevent accidental transfer of the gel to other people.
- Wait at least 2 hoursbefore showering, swimming, or bathing.
- Wash the application site with soap and water before any situation where contact between the application site and another person is likely.
Using more Testavan than prescribed
If more Testavan than prescribed is used by mistake, wash the application site with soap and water as soon as possible.
Use only the amount of medicine prescribed by your doctor. Symptoms such as irritability, nervousness, weight gain, and prolonged or frequent erections may indicate the use of too much medicine.
Missing a dose of Testavan
Do not take a double dose to make up for a missed dose.
- If it is less than 12 hours until the next dose, do not take the missed dose, and take the next dose as scheduled.
- If it is more than 12 hours until the next dose, take the missed dose. Continue treatment as usual the next day.
Stopping treatment with Testavan
Before stopping treatment with Testavan, discuss it with your doctor.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common (may affect up to 1 in 10 people):
- skin irritation at the site of application (including rash, redness, dryness, itching),
- high levels of fats (triglycerides) in the blood,
- increased blood pressure,
- increased levels of prostate-specific antigen (PSA). PSA is a protein produced by the prostate gland that can be used to detect prostate diseases,
- increased hematocrit (red blood cell count), detected by regular blood tests.
Uncommon (may affect up to 1 in 100 people):
- increased levels of hemoglobin (a component of red blood cells that carries oxygen), detected by regular blood tests,
- headache.
Other known side effects of testosterone treatment include:
acne, seborrhea, hair loss, sweating, excessive hair growth on the body, itching or numbness of the skin, headache, dizziness, nausea, hot flashes, fluid retention (such as swelling of the ankles), weight gain, sleep apnea, shortness of breath, allergic reactions, malaise, mood changes (such as aggression, hostility, nervousness, anxiety, depression),
insomnia, muscle pain or cramps, breast enlargement, increased red blood cell count, decreased red blood cell count, blood clots in blood vessels, jaundice (liver problems that can sometimes be associated with yellowing of the skin and eyes),
abnormal liver function test results.
Changes in sex drive, increased erections, priapism (prolonged and painful erections), testicular disorders, decreased sperm count, difficulty urinating, changes in the prostate gland, prostate cancer (there is no conclusive evidence that testosterone replacement therapy in men with hypogonadism causes prostate cancer, but testosterone treatment should be avoided in men with diagnosed or suspected prostate cancer).
Long-term administration of testosterone may cause changes in electrolyte and water balance in the body.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Aleje Jerozolimskie 181C, 02-222 Warsaw, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309,
e-mail: [email protected]
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Testavan
Keep the medicine out of sight and reach of children.
There are no special storage instructions for this medicine.
Do not use this medicine after the expiry date stated on the label of the pump actuator and the carton after EXP. The expiry date refers to the last day of the month.
The batch number is stated after the LOT.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Testavan contains
The active substance of the medicine is testosterone.
The other ingredients are: ethanol 96%, propylene glycol, diethylene glycol monoethyl ether, carbomer 980, tromethamine, disodium edetate, purified water.
One gram of gel contains 20 mg of testosterone. One pump actuation delivers 1.15 g of gel (1.25 ml) containing 23 mg of testosterone.
What Testavan looks like and contents of the pack
Testavan is a homogeneous, translucent to slightly opalescent gel.
The following pack sizes are available with an applicator and hygiene cap or without:
Single pack containing 1 pump actuator.
Bulk packs containing 3 pump actuators.
One pump actuator contains 85.5 g of Testavan gel and allows for 56 measured doses.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
The Simple Pharma Company Limited
Ground Floor, 71 Lower Baggot St
Dublin D02 P593
Ireland
Manufacturer:
Copea Pharma Europe Limited,
Medici House, Unit 2, Ashbourne Manufacturing Park,
Ashbourne, Co. Meath, A84 KH58,
Ireland
CAP applicator:
The Simple Pharma Company Limited
Ground Floor, 71 Lower Baggot St
Dublin D02 P593
Ireland
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Testavan: Austria, Bulgaria, Denmark, Estonia, Finland, France, Spain, Netherlands, Iceland,
Liechtenstein, Lithuania, , Germany, Norway, Poland, Portugal, Czech Republic,
Romania, Slovakia, Sweden, United Kingdom, Italy, United Kingdom (Northern Ireland)
Testarzon: Belgium, Croatia, , Greece, Ireland, Luxembourg, Latvia, Slovenia, Hungary
Date of last revision of the leaflet: August 2023
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterCopea Pharma Europe Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TestavanDosage form: Gel, 25 mg/2.5 gActive substance: testosteronePrescription not requiredDosage form: Gel, 50 mg/5 gActive substance: testosteronePrescription not requiredDosage form: Gel, 16.2 mg/gActive substance: testosteronePrescription required
Alternatives to Testavan in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Testavan in Spain
Alternative to Testavan in Ukraine
Online doctors for Testavan
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Testavan – subject to medical assessment and local rules.











