How to use Temsirolimus Accord
Package Leaflet: Information for the Patient
Temsirolimus Accord, 30 mg, Concentrate and Solvent for Solution for Infusion
Temsirolimus
Read All of This Leaflet Carefully Before Using This Medicine.
- Keep This Leaflet. You May Need to Read It Again.
- If You Have Any Further Questions, Ask Your Doctor, Pharmacist, or Nurse.
- If You Get Any Side Effects, Talk to Your Doctor, Pharmacist, or Nurse. This Includes Any Possible Side Effects Not Listed in This Leaflet. See Section 4.
Contents of the Pack
- 1. What Temsirolimus Accord Is and What It Is Used For
- 2. Before You Use Temsirolimus Accord
- 3. How to Use Temsirolimus Accord
- 4. Possible Side Effects
- 5. How to Store Temsirolimus Accord
- 6. Contents of the Pack and Other Information
1. What Temsirolimus Accord Is and What It Is Used For
Temsirolimus Accord Contains the Active Substance Temsirolimus.
Temsirolimus Is a Selective Inhibitor of the Mtor (Kinase) Enzyme That Blocks the Growth and Division of Cancer Cells.
Temsirolimus Accord Is Used to Treat Adult Patients with Advanced Kidney Cancer.
- Advanced Kidney Cancer.
2. Before You Use Temsirolimus Accord
When Not to Use Temsirolimus Accord
- If You Are Allergic to Temsirolimus, Polysorbate 80, or Any of the Other Ingredients of Temsirolimus Accord (Listed in Section 6),
- If You Are Allergic to Sirolimus (Used to Prevent Transplanted Kidney Rejection), Because Sirolimus Is Released from Temsirolimus in the Body,
- If You Have Mantle Cell Lymphoma and Liver Disease.
Warnings and Precautions
Before Starting Treatment with Temsirolimus Accord, Discuss with Your Doctor, Pharmacist, or Nurse:
- If You Are Allergic to Antihistamine Medicines or Cannot Take Antihistamine Medicines for Other Medical Reasons. Antihistamine Medicines Are Given to Prevent an Allergic Reaction to Temsirolimus Accord, Including Life-Threatening or Rare Allergic Reactions That Can Cause Death. You Should Discuss Alternatives with Your Doctor.
- If You Have or Have Had Brain Tumors or Spinal Cord Tumors, Bleeding or Bruising Problems, or Are Taking Medicines That Prevent Blood Clotting (Such as Warfarin or Acenocoumarol). Temsirolimus Accord May Increase the Risk of Bleeding in the Brain. If You Are Taking Blood-Thinning Medicines or Have Any Bleeding or Bruising While Taking Temsirolimus Accord, Tell Your Doctor.
If You Have a Fever, Cough, and (or) Shortness of Breath, Temsirolimus Accord May Weaken Your Immune System. While Taking Temsirolimus Accord, You May Be at Increased Risk of Infection of the Blood, Skin, Upper Respiratory Tract (Including Pneumonia), and (or) Urinary Tract. If You Develop New Symptoms or Worsening of Symptoms, or If You Are Currently Taking or Have Recently Taken Medicines That Weaken Your Immune System, Tell Your Doctor.
- If You Have or Have Had Pneumonia. Temsirolimus Accord May Cause Non-Specific Interstitial Pneumonia. Some Patients May Not Have Symptoms or May Have Mild Symptoms. For This Reason, Your Doctor May Recommend a Computed Tomography (CT) Scan or Chest X-Ray Before and During Treatment with Temsirolimus Accord. If You Develop Any New Symptoms or Worsening of Respiratory Symptoms, Such as Shortness of Breath or Difficulty Breathing, Tell Your Doctor Immediately.
- If You Drink Alcohol or Are an Alcoholic. Temsirolimus Accord Contains Alcohol and May Be Harmful to People Who Drink Alcohol or Have Alcoholism. If You Abuse Alcohol or Drink Alcohol (See "Temsirolimus Accord Contains Ethanol"), Tell Your Doctor.
- If You Have or Have Had Kidney Disease. Your Doctor Will Monitor Your Kidney Function.
- If You Have or Have Had Liver Disease. If You Develop Any of the Following Symptoms While Taking Temsirolimus Accord: Itching, Yellowing of the Whites of the Eyes or Skin, Dark Urine, and Pain or Discomfort in the Right Upper Abdomen, Tell Your Doctor. Your Doctor May Order a Blood Test to Check Your Liver Function and May Decide to Reduce the Dose of Temsirolimus Accord.
- If You Have or Have Had High Cholesterol Levels. Temsirolimus Accord May Increase Triglyceride and (or) Cholesterol Levels, Which May Require Treatment with Lipid-Lowering Medicines.
- If You Are Scheduled for Surgery or Have Recently Had Surgery. Temsirolimus Accord May Increase the Risk of Wound Healing Problems. Usually, Temsirolimus Accord Is Stopped Before Surgery. Your Doctor Will Decide When You Can Restart Treatment with Temsirolimus Accord.
- If You Are Scheduled for Vaccination While Taking Temsirolimus Accord. The Effectiveness of the Vaccine May Be Reduced; You Should Avoid Taking Certain Vaccines While Taking Temsirolimus Accord.
- If You Are Over 65 Years Old. You May Be More Likely to Experience Certain Side Effects, Including Facial Swelling, Diarrhea, Pneumonia, Anxiety, Depression, Shortness of Breath, Reduced White Blood Cell Count, Muscle Pain, Taste Disturbances, Upper Respiratory Tract Infection, Fluid Around the Lungs, Ulceration, and Mouth and (or) Gastrointestinal Tract Inflammation, Common Cold, Dizziness, and Infections.
- Temsirolimus Accord May Increase Blood Sugar Levels and Worsen Diabetes Symptoms. This May Lead to the Need for Insulin and (or) Oral Anti-Diabetic Medicines. If You Experience Excessive Thirst or Increased Frequency and Amount of Urine, Tell Your Doctor.
- Temsirolimus Accord May Reduce the Number of Blood Cells That Help the Blood to Clot and Fight Infections. This May Increase the Risk of Bleeding and (or) Infections (See "Possible Side Effects").
- If You Have or Have Had Eye Problems, Such as Cataracts. Your Doctor May Recommend an Eye Examination Before and During Treatment with Temsirolimus Accord.
- Patients Taking Temsirolimus Accord May Be at Increased Risk of Developing Certain Types of Cancer, Such as Skin Cancer and Lymphoma.
- Patients Taking Temsirolimus Accord May Be at Increased Risk of Heart Attack. If You Experience Symptoms Such as Chest Pain or Tightness, Arm, Back, or Jaw Pain, Shortness of Breath, Nausea, Anxiety, Sweating, or Dizziness, Tell Your Doctor.
If You Have Any Further Questions, Ask Your Doctor, Pharmacist, or Nurse.
Children and Adolescents
This Medicine Is Not Intended for Use in Children and Adolescents Under 18 Years Old, as Advanced Kidney Cancer Does Not Occur in This Patient Population, and the Medicine Is Not Effective for Other Types of Cancer.
Temsirolimus Accord with Other Medicines
Tell Your Doctor or Pharmacist About All Medicines You Are Taking or Have Recently Taken.
Certain Medicines May Affect the Breakdown or Metabolism of Temsirolimus Accord and May Require Dose Adjustment of Temsirolimus Accord. In Particular, Inform Your Doctor or Pharmacist If You Are Taking:
- HIV Protease Inhibitors,
- Antibiotics (Including Rifampicin) or Antifungal Medicines (Including Itraconazole, Ketoconazole, and Voriconazole) Used to Treat Infections,
- Nefazodone or Selective Serotonin Reuptake Inhibitors Used to Treat Depression,
- Anti-Epileptic Medicines, Including Carbamazepine, Phenytoin, and Phenobarbital,
- Rifabutin Used to Treat Infections in HIV-Infected Patients and Other Diseases,
- Herbal or Natural Medicines Containing St. John's Wort (Hypericum Perforatum) Used to Treat Mild Depression,
- Angiotensin-Converting Enzyme Inhibitors (Such as Enalapril, Ramipril, Lisinopril) or Calcium Channel Blockers (Such as Amlodipine) Used to Treat High Blood Pressure or Other Heart and Blood Vessel Problems,
- Amphiphilic Medicines Used to Treat Heart Rhythm Disorders (Such as Amiodarone) or Statins Used to Treat High Cholesterol,
- Sunitinib Used to Treat Kidney Cancer,
- Medicines That Are Substrates of P-Glycoprotein (Such as Digoxin, Vinblastine, Colchicine, Dabigatran, Lenalidomide, Paclitaxel).
Temsirolimus Accord with Food and Drink
Grapefruit and Grapefruit Juice May Increase the Levels of Temsirolimus Accord in the Blood, and Therefore Should Not Be Consumed.
Pregnancy and Breastfeeding
If You Are Pregnant or Breastfeeding, Think You May Be Pregnant, or Are Planning to Have a Baby, Ask Your Doctor for Advice Before Taking This Medicine.
Temsirolimus Accord Has Not Been Studied in Pregnant Women and Should Not Be Used During Pregnancy Unless Clearly Necessary.
During Treatment with Temsirolimus Accord, Women of Childbearing Age Should Avoid Becoming Pregnant by Using Effective Contraception. Male Patients with Female Partners of Childbearing Age Should Use Effective Contraception During Treatment with Temsirolimus Accord.
During Treatment with Temsirolimus Accord, You Should Not Breastfeed, as This Medicine May Affect the Growth and Development of Your Baby.
Temsirolimus Accord Contains Alcohol (Ethanol). If You Are Pregnant or Breastfeeding, Ask Your Doctor or Pharmacist for Advice Before Taking This Medicine.
Temsirolimus Accord Contains Propylene Glycol. If You Are Pregnant, You Should Not Take This Medicine Unless Advised by Your Doctor (See "Temsirolimus Accord Contains Propylene Glycol").
Propylene Glycol May Pass into Breast Milk. If You Are Breastfeeding, You Should Not Take This Medicine Unless Advised by Your Doctor (See "Temsirolimus Accord Contains Propylene Glycol").
Driving and Using Machines
It Is Unlikely That Temsirolimus Accord Will Affect Your Ability to Drive or Use Machines. However, Very Common Side Effects of This Medicine Include Nausea and Vomiting and Sleep Disturbances. If You Experience Nausea and Vomiting or Sleep Disturbances, You Should Be Cautious While Driving or Using Machines.
Temsirolimus Accord Contains Ethanol (Alcohol)
This Medicine Contains 474 Mg of Ethanol (Alcohol) in the Concentrate Vial (395 Mg/Ml, 39.5% W/V) and 358.2 Mg of Ethanol (Alcohol) in the Solvent Vial (199.0 Mg/Ml, 19.90% W/V), Which Is Equivalent to 18 Ml of Beer or 7 Ml of Wine per 25 Mg Dose. This Is Harmful to Patients with Alcohol Dependence and Should Be Taken into Account in Pregnant or Breastfeeding Women and in Patients at Risk, Such as Those with Liver Disease or Epilepsy.
The Amount of Alcohol in This Medicine Is Unlikely to Have an Effect on Adults.
If You Are Alcohol-Dependent, Ask Your Doctor or Pharmacist for Advice Before Taking This Medicine.
The Amount of Alcohol in This Medicine May Affect the Effects of Other Medicines. If You Are Taking Other Medicines, Ask Your Doctor or Pharmacist for Advice.
The Amount of Alcohol in This Medicine May Affect Your Ability to Drive or Use Machines or Alter the Effects of Other Medicines (See "Warnings and Precautions" and "Driving and Using Machines").
Temsirolimus Accord Contains Propylene Glycol
Temsirolimus Accord Contains 503.0 Mg of Propylene Glycol in Each 25 Mg Dose, Which Is Equivalent to 201.2 Mg/Ml of the Diluted Medicine. Before Administering the Medicine to a Child Under 5 Years Old, Consult a Doctor or Pharmacist, Especially If the Child Is Taking Other Medicines That Contain Propylene Glycol or Alcohol. Pregnant or Breastfeeding Women and Patients with Liver or Kidney Problems Should Not Take This Medicine Unless Advised by a Doctor. The Doctor May Decide to Perform Additional Tests on These Patients.
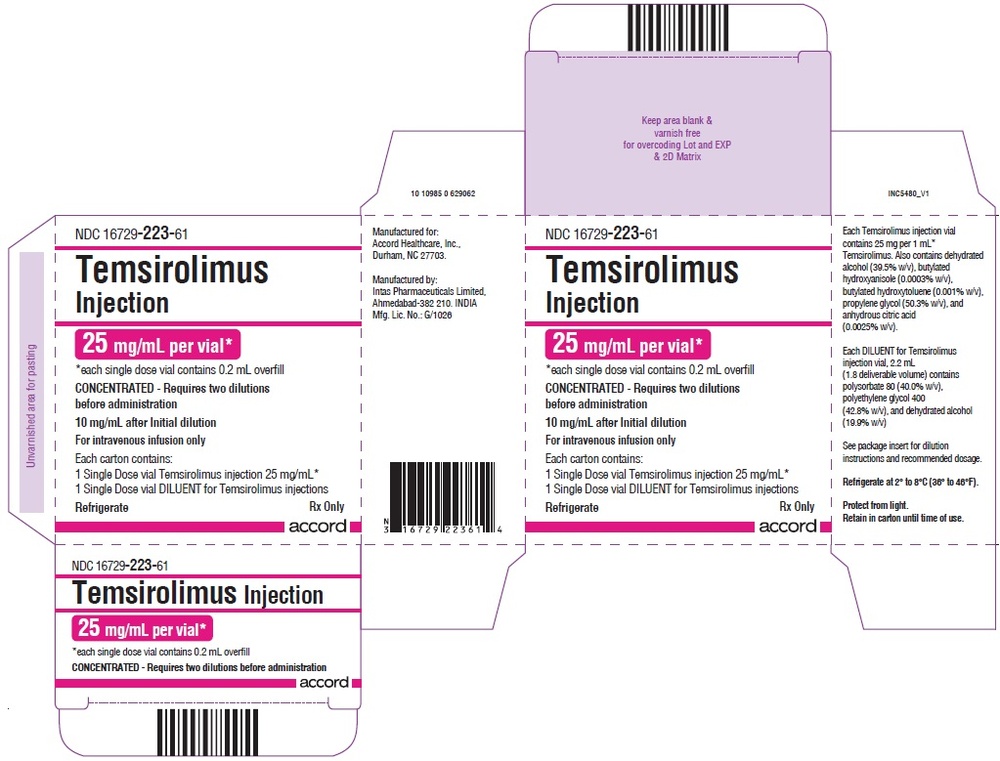
3. How to Use Temsirolimus Accord
Temsirolimus Accord Will Always Be Prepared and Administered by a Doctor or Other Qualified Healthcare Professional.
You Should Receive an Antihistamine Medicine (to Prevent an Allergic Reaction to Temsirolimus Accord) Intravenously About 30 Minutes Before Administration of Temsirolimus Accord.
To Obtain a Concentration of 10 Mg/Ml, the Temsirolimus Accord Concentrate Should Be Diluted in 1.8 Ml of the Provided Solvent Before Administration in a Sodium Chloride 9 Mg/Ml (0.9%) Solution for Injection (Instructions for Dilution Are at the End of This Leaflet).
The Recommended Dose for the Treatment of Kidney Cancer Is 25 Mg Administered Intravenously Over a Period of 30 to 60 Minutes Once a Week.
Treatment with Temsirolimus Accord Should Be Continued Until the Patient No Longer Benefits from Therapy or Until Severe Side Effects Occur.
Since This Medicine Is Prepared and Administered by a Healthcare Professional, It Is Unlikely That You Will Receive Too Much of the Medicine or Miss a Dose.
If You Have Any Further Questions on the Use of This Medicine, Ask Your Doctor, Pharmacist, or Nurse.
4. Possible Side Effects
Like All Medicines, This Medicine Can Cause Side Effects, Although Not Everybody Gets Them.
The Most Important Side Effects That May Occur During Treatment with Temsirolimus Accord Are Listed Below. If You Experience Any of These, You Should Go to the Emergency Department Immediately.
Allergic Reactions
If You Experience Symptoms of Angioedema, Such as Swelling of the Face, Tongue, or Throat, and Difficulty Breathing, Tell Your Doctor or Nurse Immediately.
If You Experience Any of These Symptoms During Administration of Temsirolimus Accord, Your Doctor or Nurse Will Stop the Infusion.
Bleeding in the Brain
Go to the Emergency Department ImmediatelyIf You Are Disoriented, Extremely Tired, Have Difficulty Speaking or Swallowing, and Your Pupils Are of Different Sizes. These Symptoms May Be Caused by Bleeding in the Brain.
Perforation, Rupture, or Ulceration of the Intestine
Go to the Emergency Department ImmediatelyIf You Experience Abdominal Pain, High Fever, Nausea and Vomiting, or Blood in Your Stool. These Symptoms May Be Caused by a Hole in the Intestine.
Kidney Failure
Go to the Emergency Department ImmediatelyIf You Experience Generalized Swelling, Shortness of Breath, or Fatigue. These Symptoms May Be Caused by Sudden Kidney Failure.
Blood Clots in the Lungs
Go to the Emergency Department ImmediatelyIf You Experience Shortness of Breath, Chest Pain, Coughing Up Blood, Rapid Heart Rate, Nausea, Fainting, Sweating, or Dizziness. These Symptoms May Be Caused by a Blood Clot in the Lungs.
You Should Also Tell Your Doctor Immediately
- If You Experience a Cough, Chest Pain, or Difficulty Breathing. Your Doctor May Recommend a Chest X-Ray.
- If Your White Blood Cell Count Decreases. This May Increase the Risk of Fever and Infection.
- If Your Platelet Count Decreases (a Type of Blood Cell That Helps the Blood to Clot). This May Increase the Risk of Bleeding in the Body.
- If Your Cholesterol or Triglyceride Levels Increase in the Blood.
- If You Experience Excessive Thirst or Increased Frequency and Amount of Urine. Your Doctor May Recommend Treatment with Insulin and (or) Oral Anti-Diabetic Medicines.
- If You Have Recently Had Surgery. Your Doctor May Delay Administration of Temsirolimus Accord Until the Wound Has Fully Healed, as This Medicine May Interfere with the Wound Healing Process.
Other Side Effects of Temsirolimus Accord May Include
Very Common Side Effects (May Affect More Than 1 in 10 People)
General Feeling of Weakness, Chills, Swelling Due to Fluid Retention, Pain (Including Abdominal, Back, Chest, or Joint Pain), Nausea and Vomiting, Diarrhea, Constipation, Headache, Fever, Herpes Simplex Infection, and Inflammation of the Mouth and (or) Gastrointestinal Tract, Cough, Pneumonia, Nosebleeds, Rash, Itching, Dry Skin, Decreased Appetite, Shortness of Breath, Low Potassium Levels in the Blood (Which May Cause Muscle Weakness), Low Red Blood Cell Count, Decreased White Blood Cell Count, Which Is Associated with an Increased Risk of Infection, High Blood Sugar Levels, High Cholesterol Levels, High Triglyceride Levels, Abscess, Infections (Including Eye Infections, Flu, Viral Infections, Bronchitis), Abnormal Kidney Function (Including Kidney Failure), Blood Tests Showing Changes in Kidney Function, Changes in Taste, Difficulty Sleeping, Low Platelet Count, Which May Cause Bleeding and Bruising.
Common Side Effects (May Affect Up to 1 in 10 People)
Cold, Redness and Swelling of the Gums, Mouth Pain (Including Mouth Ulcers), Bloating, Sore Throat, High Blood Pressure, Redness of the Eyes, Excessive Tear Production, Loss of Taste, Redness and Swelling of Hair Follicles in the Skin, Allergic Reactions, Severe Skin Peeling, Increased Blood Clotting (Including Deep Vein Thrombosis), Low Calcium Levels in the Blood, Low Phosphate Levels in the Blood, Upper Respiratory Tract Infections, Pneumonia, Fluid in the Lungs, Blood Infections, Dehydration, Agitation, Depression, Numbness and Tingling of the Skin, Dizziness, Drowsiness, Bleeding (from the Lips, Mouth, Stomach, or Intestines), Gastritis, Difficulty Swallowing, Skin Discoloration (Bruising), Small Bleeds, Nail Disorders, Acne, Fungal Infections, Urinary Tract Infections, Cysts, Blood Tests Showing Changes in Liver Function, High Levels of Other Lipids in the Blood, Diabetes, Muscle Pain.
Uncommon Side Effects (May Affect Up to 1 in 100 People)
Fluid Around the Heart (Which May Require Drainage and Affect Blood Flow).
Bleeding in the Brain in Patients with Brain Tumors or Those Receiving Anti-Clotting Treatment,
Bleeding in the Eyes.
Blood Clots in the Lungs, Hole in the Intestine, Wound Healing Problems After Surgery, Inflammation and Swelling of the Voice Box.
Rare Side Effects (May Affect Up to 1 in 1,000 People)
Pneumonia Caused by Pneumocystis Jiroveci.
Side Effects with Unknown Frequency (Cannot Be Estimated from the Available Data)
Swelling of the Face, Lips, Tongue, and Throat That May Cause Difficulty Breathing.
Severe Skin and (or) Mucous Membrane Reactions, Which May Include Painful Blistering and Fever (Stevens-Johnson Syndrome).
Muscle Pain of Unknown Cause, Sensitivity, or Weakness, Which May Indicate Muscle Damage (Rhabdomyolysis).
Reporting Side Effects
If You Experience Any Side Effects, Including Any Not Listed in This Leaflet, Tell Your Doctor or Pharmacist. You Can Also Report Side Effects Directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl. You Can Also Report Side Effects to the Marketing Authorization Holder. By Reporting Side Effects, You Can Help Provide More Information on the Safety of This Medicine.
5. How to Store Temsirolimus Accord
Keep This Medicine Out of the Sight and Reach of Children.
Do Not Use This Medicine After the Expiry Date Stated on the Label and Carton After EXP. The Expiry Date Refers to the Last Day of the Month Stated.
Store in a Refrigerator (2°C – 8°C).
Do Not Freeze.
Store the Vials in the Outer Carton to Protect from Light.
After First Dilution of the Temsirolimus Accord Concentrate with the Provided Solvent, Chemical and Physical Stability Has Been Demonstrated for 24 Hours at Temperatures Below 25°C and Protected from Light.
After Further Dilution of the Concentrate and Solvent Mixture in Sodium Chloride 9 Mg/Ml (0.9%) Solution for Injection, Chemical and Physical Stability Has Been Demonstrated for 6 Hours at Temperatures Below 25°C and Protected from Light.
From a Microbiological Point of View, the Medicine Should Be Used Immediately.
Medicines Should Not Be Disposed of via Wastewater or Household Waste. Ask Your Pharmacist How to Dispose of Medicines No Longer Required. These Measures Will Help Protect the Environment.
6. Contents of the Pack and Other Information
What Temsirolimus Accord Contains
- The Active Substance Is Temsirolimus.
Each Vial of Concentrate Contains 30 Mg of Temsirolimus.
After First Dilution of the Concentrate in 1.8 Ml of the Provided Solvent, the Concentration of Temsirolimus Is 10 Mg/Ml.
- The Other Ingredients of the Concentrate Are Ethanol Anhydrous, Butylhydroxyanisole, Butylhydroxytoluene (E321), Propylene Glycol, Citric Acid (E 330).
- The Solvent Contains Polysorbate 80 (E 433), Macrogol 400, and Ethanol Anhydrous [See "Temsirolimus Accord Contains Ethanol (Alcohol)" and "Temsirolimus Accord Contains Propylene Glycol"].
What Temsirolimus Accord Looks Like and Contents of the Pack
Temsirolimus Accord Is a Concentrate and Solvent for Solution for Infusion.
The Concentrate Is a Clear, Colorless or Pale Yellow Solution. The Solvent Is a Clear or Slightly Opaque, Pale Yellow or Yellow Solution. The Solutions Are Essentially Free from Visible Particulate Matter.
Each Pack of Temsirolimus Accord Contains One 1.2 Ml Vial of Concentrate and One 2.2 Ml Vial of Solvent.
Concentrate
The Vial Is Made of Colorless Glass (Type I) with a Butyl Rubber Stopper and an Aluminum Flip-Off Seal and a Red Flip-Off Cap, Containing 1.2 Ml of Concentrate.
Solvent
The Vial Is Made of Colorless Glass (Type I) with a Butyl Rubber Stopper and an Aluminum Flip-Off Seal and a Dark Blue Flip-Off Cap, Containing 2.2 Ml of Solvent.
Marketing Authorization Holder
Accord Healthcare Polska Sp. z o.o.
Ul. Taśmowa 7
02-677 Warsaw
Manufacturer/Importer
Accord Healthcare B.V.
Winthontlaan 200
3526KV Utrecht
Netherlands
Accord Healthcare Polska Sp. z o.o.
Ul. Lutomierska 50
95-200 Pabianice
Date of Last Revision of the Leaflet:
Information Intended for Healthcare Professionals Only
When Preparing the Medicine for Administration and Administering the Prepared Mixtures, Protect Temsirolimus Accord from Excessive Room and Sunlight.
Bags and (or) Containers That Come into Contact with Temsirolimus Accord Must Be Made of Glass or Polyolefin (e.g., Polyethylene).
Administration Sets and Medical Devices Containing Polysorbate 80 Should Not Be Used with Temsirolimus Accord, as Polysorbate 80 May Leach Di-(2-Ethylhexyl) Phthalate (DEHP) from Polyvinyl Chloride (PVC) Administration Sets and Medical Devices.
Do Not Use If Particulate Matter or Discoloration Is Observed.
Dilution
The Temsirolimus Accord Concentrate for Solution for Infusion Should Be Diluted in the Provided Solvent Before Administration in a Sodium Chloride 9 Mg/Ml (0.9%) Solution for Injection.
Note: The Contents of Each Vial of Temsirolimus Accord Must Be Diluted According to the Following Instructions. The Required Volume of the Concentrate and Solvent Mixture from Each Vial Must Be Combined in One Syringe and Quickly Injected into 250 Ml of Sodium Chloride 9 Mg/Ml (0.9%) Solution for Injection (Instructions for Dilution Are Below).
The Concentrate and Solvent Mixture Should Be Visually Inspected for Particulate Matter and Discoloration.
Do Not Use If Particulate Matter or Discoloration Is Observed.
Preparation Should Be Carried Out Under Aseptic Conditions and in Accordance with Local Guidelines for Handling Cytotoxic/Cytostatic Medicines, According to the Following Two-Step Process:
STEP 1: DILUTION OF THE CONCENTRATE FOR SOLUTION FOR INFUSION IN THE PROVIDED SOLVENT
- Withdraw 1.8 Ml of the Provided Solvent.
- Inject 1.8 Ml of Solvent into the Vial of Temsirolimus Accord 30 Mg Concentrate.
- Mix the Solvent and Concentrate Well by Rotating the Vial Upside Down. Allow Time for Any Foam to Dissolve. The Solution Should Be Clear or Slightly Opaque, Colorless, Pale Yellow, or Yellow, and Essentially Free from Visible Particulate Matter.
One Vial of Temsirolimus Accord Concentrate Contains 30 Mg of Temsirolimus: After Mixing 1.2 Ml of Concentrate with 1.8 Ml of the Provided Solvent, the Total Volume Is 3.0 Ml, with a Temsirolimus Concentration of 10 Mg/Ml. The Concentrate and Solvent Mixture Is Stable for Up to 24 Hours at Temperatures Below 25°C.
STEP 2: ADMINISTRATION OF THE CONCENTRATE AND SOLVENT MIXTURE IN SODIUM CHLORIDE 9 MG/ML (0.9%) SOLUTION FOR INJECTION
- Withdraw the Required Volume of the Concentrate and Solvent Mixture from the Vial: e.g., 2.5 Ml to Obtain a Dose of 25 Mg of Temsirolimus.
- Quickly Inject the Withdrawn Volume into 250 Ml of Sodium Chloride 9 Mg/Ml (0.9%) Solution for Injection to Ensure Good Mixing.
Mix the Prepared Mixture by Rotating the Bag or Bottle, Avoiding Excessive Agitation, Which May Cause Foaming.
The Final Diluted Solution in the Bag or Bottle Should Be Visually Inspected for Particulate Matter and Discoloration Before Administration. The Prepared Temsirolimus Accord Mixture in Sodium Chloride 9 Mg/Ml (0.9%) Solution for Injection Should Be Protected from Excessive Room and Sunlight.
Administration
- Administration of the Final Diluted Solution Should Be Completed Within 6 Hours of the Initial Dilution of Temsirolimus Accord in Sodium Chloride 9 Mg/Ml (0.9%) Solution for Injection.
- Temsirolimus Accord Is Administered as an Intravenous Infusion Over a Period of 30 to 60 Minutes Once a Week. The Preferred Method of Administration Is by Using an Infusion Pump to Ensure Accurate Delivery of the Medicine.
- In Order to Avoid Excessive Loss of the Medicinal Product and to Reduce the Degree of DEHP Extraction, Suitable Administration Materials Should Be Used. Administration Materials Should Be Made of DEHP-Free or PVC-Free Materials, Equipped with an In-Line Filter. To Avoid the Risk of Administering Particles Larger Than 5 Microns, It Is Recommended to Use a Polyethersulfone Filter with a Pore Size of 5 Microns or Less in the Administration Line. If the Infusion Set Does Not Contain an In-Line Filter, a Filter Should Be Added at the End of the Set (e.g., an End-Port Filter) Before the Mixture Enters the Patient's Vein. Different End-Port Filters with Pore Sizes from 0.2 to 5 Microns Can Be Used. It Is Not Recommended to Use Both an In-Line Filter and an End-Port Filter.
- The Diluted Solution of Temsirolimus Accord Contains Polysorbate 80 and Therefore Should Be Administered Using Materials Compatible with This Excipient. It Is Essential to Follow the Instructions in the SmPC in Sections 4.2 and 6.6.
Disposal
Any Unused Medicinal Product or Waste Material Should Be Disposed of in Accordance with Local Requirements.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAccord Healthcare B.V. Accord Healthcare Polska Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Temsirolimus AccordDosage form: Tablets, 2.5 mgActive substance: everolimusManufacturer: Synthon B.V. Synthon Hispania S.L.Prescription requiredDosage form: Tablets, 5 mgActive substance: everolimusManufacturer: Synthon B.V. Synthon Hispania S.L.Prescription requiredDosage form: Tablets, 10 mgActive substance: everolimusManufacturer: Synthon B.V. Synthon Hispania S.L.Prescription required
Alternatives to Temsirolimus Accord in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Temsirolimus Accord in Spain
Online doctors for Temsirolimus Accord
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Temsirolimus Accord – subject to medical assessment and local rules.