
Tardisol Babi
Ask a doctor about a prescription for Tardisol Babi

How to use Tardisol Babi
Leaflet attached to the packaging: patient information
Tardysol Baby, 20 mg/ml, oral solution
Ferrum asFerrosi sulfas heptahydricus
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for this person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the child experiences any side effects, including any side effects not listed in this leaflet, tell the doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Tardysol Baby and what is it used for
- 2. Important information before taking Tardysol Baby
- 3. How to take Tardysol Baby
- 4. Possible side effects
- 5. How to store Tardysol Baby
- 6. Contents of the pack and other information
1. What is Tardysol Baby and what is it used for
Tardysol Baby contains iron.
The medicine is used in children from 6 to 24 months to treat iron deficiency anemia (insufficient number of red blood cells due to insufficient iron).
2. Important information before taking Tardysol Baby
When not to give Tardysol Baby
- if the child is allergic to iron or any of the other ingredients of this medicine (listed in section 6);
- if the child has too much iron in the body;
- if the child has a type of anemia (insufficient number of red blood cells) that is not caused by iron deficiency or that causes iron overload (e.g. thalassemia, anemia resistant to treatment, anemia caused by bone marrow failure). IN CASE OF ANY DOUBTS ABOUT THIS MEDICINE, CONSULT A DOCTOR OR PHARMACIST.
Warnings and precautions
Before starting treatment with Tardysol Baby in a child, discuss it with a doctor or pharmacist.
- If the child is taking Tardysol Baby due to iron deficiency anemia, the doctor will also investigate the cause of the anemia to treat it.
- If the iron deficiency anemia in the child is related to an inflammatory disease, treatment with Tardysol Baby will not be effective.
- As literature data indicate, in elderly patients with renal failure, diabetes (abnormally high blood sugar levels) and (or) high blood pressure (hypertension), taking medications for these conditions and iron supplements for anemia, gastrointestinal wall discoloration has been reported. This color change can make surgical procedures in the gastrointestinal tract more difficult. Due to the risk, in case of planned surgery, the surgeon should be informed about the child's iron intake (see section 4.4).
- In case of accidental aspiration (aspiration in the wrong direction), the medicine may enter the child's airways. Contact of the medicine with the airways can cause injuries such as necrosis (tissue death) or bronchitis (places where air passes through the lungs) or esophagus (section connecting the mouth to the stomach). These injuries can cause bronchial narrowing. Symptoms associated with these injuries may include: persistent cough, coughing up blood and (or) feeling of shortness of breath, even if aspiration occurred several days or months before the onset of these symptoms. In case of suspected aspiration and if the child has at least one of the mentioned symptoms, you should contact a doctor or the nearest emergency department as soon as possible to perform a specialist assessment and ensure that no airway damage has occurred. To reduce the risk of aspiration, follow the administration instructions provided in section 3 of this leaflet.
Children
Not applicable.
Tardysol Baby and other medicines
Tell the doctor or pharmacist about all medicines the child is taking, has recently taken, or might take.
Some medicines should not be taken at the same time as Tardysol Baby, while others require certain changes (e.g. time of administration).
If the child is receiving iron injections, Tardysol Baby should not be given.
If the patient is taking the following medicines, a minimum of 2-hour interval should be maintained between their administration and the administration of Tardysol Baby:
antibiotics (tetracyclines, fluoroquinolones)
medicine used to treat chronic urinary tract infections (acetohydroxamic acid)
medicines used to treat HIV infection (integrase inhibitors)
medicines used to treat excessive stomach acid production: preparations containing mineral compounds, charcoal or antacids (aluminum, calcium and magnesium salts)
medicines used to treat Wilson's disease or to prevent kidney stone formation (penicillamine, trientine)
medicines used to treat thyroid diseases (levothyroxine, liotyronine)
supplements and (or) medicines containing zinc, calcium or strontium.
Using Tardysol Baby with food and drink
Foods rich in calcium may reduce iron absorption and make treatment less effective. Therefore, Tardysol Baby should be given some time before a meal or after a meal, including before or after giving dairy products.
Pregnancy, breastfeeding and fertility
Tardysol Baby is not suitable for pregnant or breastfeeding women.
For adults, and in particular for pregnant women, other oral iron solutions are available. For further information, consult a doctor or pharmacist.
Driving and using machines
Not applicable.
Tardysol Baby contains sorbitol, sodium and propylene glycol
- This medicine contains 360 mg of sorbitol in each milliliter of solution. Sorbitol (E420) is a source of fructose. If the child has previously been diagnosed with intolerance to some sugars or hereditary fructose intolerance (HFI), a rare genetic disease in which the patient's body does not break down fructose, consult a doctor before giving this medicine to the child.
- This medicine contains less than 1 mmol (23 mg) of sodium per ml, which means the medicine is considered "sodium-free".
- This medicine contains 12 mg of propylene glycol (E1520) in each milliliter of solution.
3. How to take Tardysol Baby
Always take this medicine exactly as the doctor or pharmacist has told you. If you are not sure, consult a doctor or pharmacist.
Recommended dose
The dose of Tardysol Baby for the treatment of iron deficiency:
- Children with a body weight of 7 to 10 kg: the usual dose is 7 to 20 mg once a day.
- Children with a body weight of 10 to 15 kg: the usual dose is 10 to 30 mg once a day.
The dose is measured directly with the graduated pipette (see explanatory drawings).
How to prepare and give the dose
The medicine is taken orally and should be given under adult supervision.
To reduce the risk of the medicine entering the child's airways, follow the administration instructions provided in section 3 of this leaflet before giving the medicine.
Always give the medicine to the child some time before a meal or after a meal, including before or after giving dairy products (see "Using Tardysol Baby with food and drink").
Choose the time of administration according to when the child's stomach will best tolerate the medicine.
Preparing the dose
Only use the measuring pipette provided with the packaging of Tardysol Baby.
Before use, check that the pipette is clean.
The pipette allows you to draw the oral solution from the bottle and indicates the dose (mg) to be given.
Read the dose directly from the pipette's graduation.
- 1) To open the bottle, first press the cap and then unscrew it.
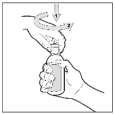
- 2) Insert the dosing pipette into the bottle, making sure the plunger is fully pressed.
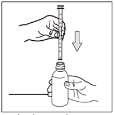
- 3) To draw the medicine, pull the pipette's plunger until the solution reaches the graduation mark closest to the prescribed dose (graduation every 5 mg). If there is an air bubble in the drawn solution, repeat the drawing step.
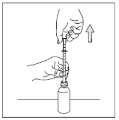
The dose indicated by the graduation will be visible above the pipette's collar.
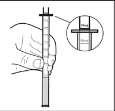
Administering the dose
Immediately after drawing the solution into the pipette, give it to the child very slowly, following the instructions provided in the card at the end of this leaflet.
To reduce the risk of the medicine entering the child's airways during administration, it is very important to strictly follow the administration instructions for young children.
Always give the medicine to the child some time before a meal or after a meal, including before or after giving dairy products.
Give the medicine directly with the pipette provided with the packaging, immediately after drawing the solution from the bottle with the pipette.
- 1) Hold the child in your arms, who is not sleeping, in a semi-sitting position with the arm bent, with the head resting on the arm.
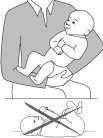
- 2) After drawing the medicine with the pipette from the bottle, immediately insert the pipette into the child's mouth, directing it towards the innerside of the cheek. Slowly press the pipette's plunger so that the medicine flows into the child's mouth one drop at a time.

Do not lay the child down immediately after giving the medicine.
Cleaning the pipette
- 1. After use, close the bottle with the cap.
- 2. Rinse the pipette several times with water, as shown in the following drawings:
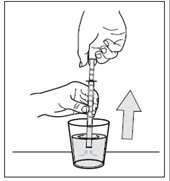
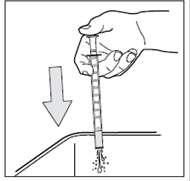
- 3. Leave the pipette to dry on a kitchen towel, then put it back in the carton with the bottle and store it out of sight and reach of children. Never leave the pipette anywhere other than the packaging or leaflet.
Duration of treatment
If the child is taking the medicine to treat iron deficiency, the duration of treatment must be sufficient to correct the deficiency and replenish the body's iron stores. Treatment may last from 3 to 6 months, or even longer, if the cause of the anemia is not effectively treated.
Administration of a higher dose of Tardysol Baby than recommended
If the child has taken a much higher dose of Tardysol Baby, contact a doctor or the nearest emergency department immediately.
Symptoms of iron overdose include:
severe gastrointestinal irritation, which can lead to tissue necrosis (necrosis of the gastrointestinal mucosa). The main symptoms are: abdominal pain, nausea, vomiting (sometimes with blood) and diarrhea (sometimes with black stools).
This may be accompanied by metabolic acidosis and shock with the following main symptoms: rapid breathing or shortness of breath, rapid heart rate, headache, confusion, drowsiness, fatigue, loss of appetite, stomach pain, vomiting, and sudden drop in blood pressure, which can lead to loss of consciousness with seizures (coma).
symptoms of abnormal kidney function (significant decrease in urine output) and liver function (pain in the right upper abdomen, yellowing of the skin or eyes, dark urine color).
Possible long-term consequences of overdose include narrowing of the gastrointestinal tract (stenosis), characterized by nausea, bloating, constipation, and abdominal distension.
Missing a dose of Tardysol Baby
If a dose is missed, give it to the child as soon as possible. However, if it is almost time for the next dose, wait for the next dose and continue treatment as usual.
Do not take a double dose to make up for the missed dose.
Stopping treatment with Tardysol Baby
Tardysol Baby should be taken as directed by the doctor. After stopping treatment, problems may occur.
If you have any further questions about taking this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur in the child, listed from most common to rarest:
- constipation
- diarrhea
- abdominal distension
- abdominal pain
- change in stool color
- nausea
- Less common (may occur in less than 1 in 100 people)
- throat swelling (laryngeal edema)
- abnormal stools
- indigestion
- vomiting
- gastritis
- itching
- red skin rash (erythematous rash)
Frequency not known (frequency cannot be estimated from available data):
- allergic reaction (hypersensitivity reaction)
- itchy rash (hives)
- tooth discoloration
- gastrointestinal wall discoloration (gastrointestinal melanosis) see section 2)
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell the doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl
By reporting side effects, you can help provide more information on the safety of this medicine.
Side effects can also be reported to the marketing authorization holder.
5. How to store Tardysol Baby
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the outer packaging.
The expiry date refers to the last day of the month.
After opening the bottle for the first time, the solution can be stored for 1 month. The medicine should be stored away from light, in its carton packaging.
Between administrations, always store the bottle and dosing pipette together in the box.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Tardysol Baby contains
- Each milliliter of oral solution contains 20 mg of iron ions in the form of iron sulfate heptahydrate.
- Other ingredients are: liquid sorbitol (non-crystallizing) (E420), orange flavor*, sulfuric acid, sodium propionate, sodium saccharin, purified water. * Orange flavor ingredients: acetic aldehyde, octanal, nonanal, decanal, ethyl butyrate, citronellal, water, citral, linalool, orange essential oil, propylene glycol (E1520).
What Tardysol Baby looks like and what the pack contains
- The medicine is a yellow to orange solution.
- 45 ml of oral solution in a brown glass bottle, closed with a child-resistant cap.
- The outer packaging contains a 2 ml pipette with a graduation from 5 to 30 mg (marked every 5 mg), allowing for oral administration
This medicine is authorized in the Member States of the European Economic Area under the following names:
Belgium
Tardyneo
France
Tardyferon
Netherlands
Tardyneo
Luxembourg
Tardyneo
Marketing authorization holder
Pierre Fabre Medicament
Les Cauquillous
81500 Lavaur, France
Manufacturer
Pierre Fabre Medicament Production
Site Progipharm
Rue du Lycée
45500 Gien, France
To obtain more detailed information about this medicine, contact the local representative of the marketing authorization holder:
Pierre Fabre Medicament Polska Sp. z o.o.
ul. Belwederska 20/22
00-762 Warsaw
tel. 22 559 63 60
Date of last revision of the leaflet: December 2024
Administration instruction card
Tardysol Baby, 20 mg/ml, oral solution
Ferrum asFerrosi sulfas heptahydricus
For children from 6 to 24 months
To reduce the risk of aspiration during administration with the dosing pipette, strictly follow all the described actions related to administering the medicine:
Give the medicine some time before a meal or after a meal, including before or after giving dairy products.
1/ Using the measuring pipette provided with the packaging, draw the solution from the bottle to the graduation mark closest to the prescribed dose (graduation every 5 mg). Do not use anything other than the dosing pipette provided in the box.
2/ Hold the child in your arms, who is not sleeping, in a semi-sitting position with the arm bent, with the head resting on the arm.
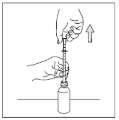
3/ Insert the pipette with the medicine into the child's mouth, directing it towards the innerside of the cheek. Slowly press the pipette's plunger so that the medicine flows into the child's mouth one drop at a time.
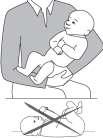

Warning: Do not lay the child down immediately after giving the medicine.
There is a risk of chokingthe child while taking the medicine, which can cause the liquid to accidentally enter their airways. If you notice the following symptoms:
cough, discomfort, pallor, cyanosis of the lips, fingers or toes, breathing difficulties or
apnea (cessation of breathing), lack of movement, loss of energy or agitation,
hold the child in a sitting position and immediately contact the emergency services. First, call 999 or 112.
The emergency services will tell you what to do to provide the child with immediate help.
After use, rinse the dosing pipette and let it dry, then put it back in the box.
If you notice any side effects in the child while taking Tardysol Baby, consult a doctor or pharmacist. Side effects can also be reported through the website: https://smz.ezdrowie.gov.pl.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterPierre Fabre Medicament Production
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tardisol BabiDosage form: Tablets, 100 mg Fe(II) + 60 mgActive substance: ferrous sulfateManufacturer: Egis Pharmaceuticals PLCPrescription requiredDosage form: Tablets, 100 mg Fe(II) + 60 mgActive substance: ferrous sulfatePrescription requiredDosage form: Tablets, 100 mg Fe(II) + 60 mgActive substance: ferrous sulfatePrescription required
Alternatives to Tardisol Babi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tardisol Babi in Ukraine
Alternative to Tardisol Babi in Spain
Online doctors for Tardisol Babi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tardisol Babi – subject to medical assessment and local rules.














