
Taflotan Multi

Ask a doctor about a prescription for Taflotan Multi

How to use Taflotan Multi
PATIENT INFORMATION LEAFLET
Leaflet accompanying the packaging: patient information
Taflotan Multi, 15 micrograms/ml, eye drops, solution
Tafluprost
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Taflotan Multi and what is it used for
- 2. Important information before using Taflotan Multi
- 3. How to use Taflotan Multi
- 4. Possible side effects
- 5. How to store Taflotan Multi
- 6. Contents of the pack and other information
1. What is Taflotan Multi and what is it used for
What kind of medicine is it and how does it work?
Taflotan Multi eye drops contain tafluprost, which belongs to a group of medicines called prostaglandin analogues. Taflotan Multi lowers the pressure in the eye. This medicine is used when the pressure in the eye is too high.
What is this medicine used for?
Taflotan Multi is used to treat a type of glaucoma called open-angle glaucoma and a condition called ocular hypertension in adults. Both of these conditions are associated with increased pressure within the eyeball and can lead to vision disturbances.
2. Important information before using Taflotan Multi
When not to use Taflotan Multi
- if you are allergic to tafluprost or any of the other ingredients of this medicine (listed in section 6)
Warnings and precautions
Before starting to use Taflotan Multi, discuss it with your doctor, pharmacist, or nurse.
Considerthat Taflotan Multi may cause the following effects, some of which may be permanent:
- Taflotan Multi may increase the length, thickness, color intensity, and/or number of eyelashes, and may cause abnormal hair growth on the eyelids.
- Taflotan Multi may cause darkening of the skin around the eyes. Wipe off any solution that remains on the skin after administration to reduce the risk of skin darkening.
- Taflotan Multi may change the color of the iris (the colored part of the eye). If Taflotan Multi is used in only one eye, the color of the treated eye may change permanently and differ from the other eye.
- Taflotan Multi may cause hair growth in areas where the solution comes into repeated contact with the skin.
Tell your doctor
- if you have kidney disease;
- if you have liver disease;
- if you have asthma;
- if you have other eye conditions.
Children and adolescents
Taflotan Multi is not recommended for children and adolescents under 18 years of age due to a lack of data on efficacy and safety.
Taflotan Multi and other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and about any medicines you plan to take.
If you are using other eye medicines, leave at least a 5-minute interval between administering Taflotan Multi and the other medicine.
Pregnancy, breastfeeding, and fertility
If you may become pregnant, you must use effective contraceptive methods during treatment with Taflotan Multi. Do not use Taflotan Multi if you are pregnant or breastfeeding. Consult your doctor.
Driving and using machines
Taflotan Multi has a minor effect on the ability to drive and use machines.
For a while after administering Taflotan Multi, vision may be blurred. Do not drive or operate machinery until your vision has cleared.
Taflotan Multi contains phosphates
This medicine contains about 0.04 mg of phosphates per drop, which corresponds to 1.2 mg/ml.
In patients with severe damage to the transparent front part of the eye (cornea), phosphates may, in very rare cases, cause corneal clouding due to calcium accumulation during treatment.
3. How to use Taflotan Multi
Always use this medicine exactly as your doctor or pharmacist has told you.
In case of doubt, consult your doctor or pharmacist.
Recommended dose of Taflotan Multiis one drop into the eye or eyes once daily in the evening. Do not use more drops or use them more often than your doctor has prescribed, as this may reduce the effect of Taflotan Multi.
Use Taflotan Multi in both eyes only on the advice of your doctor.
Taflotan Multi is for use in the eye only. Do not swallow.
Instructions for use:
When using the bottle for the first time, before administering the medicine to the eye, the patient should practice using the bottle away from the eye, squeezing it slowly so that one drop comes out.
If the patient is sure they can administer one drop, they should choose a position that is most comfortable for administering the medicine (they can sit, lie on their back, or stand in front of a mirror).
When opening a new bottle:
If the plastic ring around the neck of the bottle is missing or damaged, do not use the bottle. Record the date of opening the bottle in the space provided on the outer carton.
Every time you administer Taflotan Multi:
- 1. Wash your hands.
- 2. When opening the bottle for the first time, you must
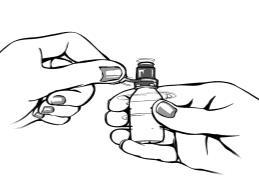
remove the protective ring from the tip
by pulling the protection.
- 3. Open the bottle by pulling the cap.
- 4.
When opening the bottle for the first time,
discard the first drop of the medicine.
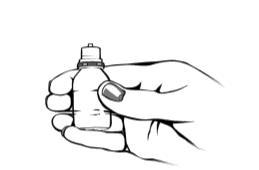
- 5. Hold the bottle between your thumb and middle finger.
- 6. Tilt your head back or lie down. Place your hand on your forehead. Your index finger should be along your eyebrow or the base of your nose. Be careful not to let the tip of the bottle touch the eye, the skin around the eye, or your fingers, to avoid potential contamination of the solution.
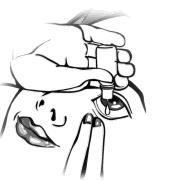
- 7. With your other hand, pull the lower eyelid down and look up. Gently squeeze the container to release a single drop into the space between the lower eyelid and the eye. Note that there may be a slight delay between squeezing the bottle and the drop appearing. Do not squeeze too hard.
- 8. Close your eye and press the inner corner of your

eye for about one minute. This helps prevent the drop from draining through the tear duct.
- 9. Wipe off any excess solution from the skin around the eyes to reduce the risk of skin darkening.
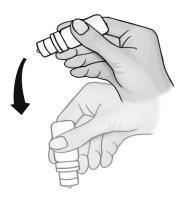
- 10. Shake the bottle once downwards to remove any remaining solution from the tip of the dropper. Do not touch or wipe the dropper.
- 11. Put the cap back on and close the bottle tightly.
About 1 ml of solution will remain, which cannot be used.
Do not try to empty the bottle.
If a drop misses the eye,try again.
If your doctor has instructed you to use drops in both eyes, repeat steps 6 to 9 for the other eye.
If you are using other eye medicines, leave at least a 5-minute interval between administering Taflotan Multi and the other medicine.
Using more Taflotan Multi than prescribedshould not cause serious side effects. Take the next dose at the usual time.
If the medicine is accidentally swallowed, contact your doctor.
Missing a dose of Taflotan Multi.
Use one drop in the eye or eyes as soon as you remember, and then continue with your normal dosing schedule. Do not use a double dose to make up for a missed dose.
Do not stop using Taflotan Multi without consulting your doctor. Stopping
use of Taflotan Multican lead to a return of increased pressure in the eye, which may cause permanent damage to the eye.
If you have any further questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects are not serious.
Common side effects
The following side effects may affect up to 1 in 10 people treated:
- headache
Eye disorders:
- eye itching
- eye irritation
- eye pain
- eye redness
- changes in length, thickness, and number of eyelashes
- dry eye
- feeling of a foreign body in the eye
- change in eyelash color
- eyelid redness
- punctate keratitis (inflammation of the cornea)
- increased sensitivity to light
- excessive tearing
- blurred vision
- reduced visual acuity
- change in iris color (may be permanent).
Uncommon side effects
The following side effects may affect up to 1 in 100 people treated:
- change in skin color around the eyes
- eyelid swelling
- eye fatigue
- conjunctival edema
- eye discharge
- blepharitis (inflammation of the eyelids)
- signs of inflammation within the eye
- eye discomfort
- conjunctival discoloration
- conjunctival follicles
- allergic conjunctivitis
- altered sensation in the eye.
Skin and subcutaneous tissue disorders:
- abnormal hair growth on the eyelids
Frequency not known: cannot be estimated from the available data
Eye disorders:
- uveitis (inflammation of the uvea, the middle layer of the eye)
- sunken eyes
- macular edema (swelling of the macula, the part of the retina responsible for central vision, leading to vision impairment).
Respiratory, thoracic, and mediastinal disorders:
- worsening of asthma, shortness of breath
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw,
phone: +48 22 49 21 301, fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Taflotan Multi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after EXP. The expiry date refers to the last day of that month.
Store in a refrigerator (2°C – 8°C). Do not freeze.
After opening: store below 25°C.
Keep the bottle in the outer carton in order to protect from light.
To prevent infection of the solution, the bottle should be discarded 3 months after
first opening and a new bottle should be used.A 3 ml bottle is for 1 month of use, a 5 ml bottle for 2 months, and a 7 ml bottle for 3 months.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Taflotan Multi contains
- -The active substance is tafluprost. 1 ml of solution contains 15 micrograms of tafluprost. One drop contains approximately 0.45 micrograms of tafluprost.
- - The other ingredientsare: glycerol, sodium dihydrogen phosphate dihydrate, disodium edetate, polysorbate 80, and water for injections. Hydrochloric acid and/or sodium hydroxide are added to adjust the pH.
What Taflotan Multi looks like and contents of the pack
Taflotan Multi is a clear, colorless solution (solution), practically free from visible particles. Taflotan Multi is available in a pack containing 1 transparent plastic bottle of 3 ml, 5 ml, or 7 ml solution or 3 transparent plastic bottles containing 3 ml solution each. The plastic bottles are closed with caps.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finland
Tubilux Pharma S.P.A.
Via Costarica 20/22
00071 Pomezia (RM)
Italy
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
| Germany | TAFLOTAN sine |
| Denmark, Finland, Iceland, Norway, Sweden | Taflotan sine |
| Bulgaria, Cyprus, Czech Republic, Estonia, Greece, Spain, Lithuania, Latvia, Portugal, Slovakia, Hungary | Taflotan |
| Poland | Taflotan Multi |
| Austria, Belgium, Croatia, Ireland, Luxembourg, Netherlands, Romania, Slovenia, United Kingdom (Northern Ireland) | Saflutan |
| Italy | Safluround |
Date of last revision of the leaflet:07/2022
Detailed information on this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterSanten OY Tubilux Pharma S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Taflotan MultiDosage form: Drops, 15 mcg/mlActive substance: tafluprostManufacturer: Santen OYPrescription not requiredDosage form: Drops, 50 mcg/mlActive substance: latanoprostManufacturer: Pharmaselect International Beteiligungs GmbHPrescription requiredDosage form: Drops, 0.3 mg/mlActive substance: bimatoprostPrescription required
Alternatives to Taflotan Multi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Taflotan Multi in Ukraine
Alternative to Taflotan Multi in Spain
Online doctors for Taflotan Multi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Taflotan Multi – subject to medical assessment and local rules.














