

Sisten 50

Ask a doctor about a prescription for Sisten 50

How to use Sisten 50
Package Leaflet: Information for the User
SYSTEN 50
3.2 mg, transdermal system, patch
Estradiol
Read the package leaflet carefully before using the medicine, as it contains important information for you.
- You should keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet:
1. What is Systen 50 and what is it used for.
- 2. Important information before using Systen 50.
- 3. How to use Systen 50.
- 4. Possible side effects.
- 5. How to store Systen 50.
- 6. Contents of the pack and other information.
1. What is Systen 50 and what is it used for
Systen 50 is a hormone replacement therapy (HRT) medicine. It contains estradiol (a female hormone belonging to estrogens) and is a transdermal system in the form of a patch, for application to the skin.
Estradiol in Systen 50 is identical to the hormone naturally produced by the ovaries in women before menopause.
Systen 50 is used to:
relieve symptoms associated with menopause
During menopause, the amount of estrogen produced in a woman's body decreases, which can cause symptoms such as a feeling of heat on the face, neck, and chest ("hot flashes"), sleep problems, irritability, and depression. Systen 50 alleviates these symptoms associated with menopause.
Systen 50 may be prescribed to a patient only if symptoms significantly interfere with her daily life.
prevent osteoporosis
After menopause, some women develop brittle bones (osteoporosis). You should discuss all available treatment options with your doctor. If a woman is at increased risk of fractures due to osteoporosis and other medicines are not suitable, Systen 50 can be used to prevent osteoporosis after menopause.
Experience in treating women over 65 years of age is limited.
2. Important information before using Systen 50
Medical History and Regular Medical Exams
Using HRT involves risks that should be considered when a patient decides whether to use hormone replacement therapy or continue its use.
Experience in treating women with premature menopause (due to ovarian failure or surgical procedure) is limited. If a patient is experiencing premature menopause, the risk associated with HRT may differ. You should talk to your doctor.
Before starting (or resuming) HRT, the doctor should take a medical history, including a family history. The doctor may decide to perform tests, including a breast exam and/or a gynecological exam, if necessary.
If a patient starts using Systen 50, she should regularly visit her doctor for check-ups (at least once a year). During these exams, she should discuss with her doctor the benefits and risks of continuing to use Systen 50.
The patient should regularly undergo breast exams, as recommended by her doctor.
When Not to Use Systen 50
In case of any of the following conditions or in case of doubt, you should tell your doctorbefore using Systen 50.
Do not start using Systen 50:
- if you are allergic to estradiolor any of the other ingredients of this medicine (listed in section 6);
- if you have or have had breast canceror if it is suspected;
- if you have or are suspected of having an estrogen-dependent tumor(e.g., endometrial cancer);
- if you have untreated endometrial hyperplasia- endometrial hyperplasia;
- if you have bleeding from the genital tract of unknown origin;
- if you are pregnant or breastfeeding;
- if you have or have had liver disease, and liver function test results have not returned to normal;
- if you have or have had blood clots in the veins(venous thromboembolic disease) e.g., in the veins of the legs (deep vein thrombosis) or lungs (pulmonary embolism);
- if you have blood coagulation disorders(deficiency of protein C, protein S, or antithrombin);
- if you have or have had thromboembolic arterial disorders(e.g., stroke, angina pectoris, myocardial infarction);
- if you have a rare, inherited blood disorder - porphyria.
If any of the above conditions occur for the first time during the use of Systen 50, you should stop using it and consult your doctor.
Warnings and Precautions
Before starting treatment, you should inform your doctor about any of the following conditions that have occurred in the past, as they may recur or worsen during the use of Systen 50.
In such a case, your doctor may decide that you need more frequent monitoring:
- uterine fibroids;
- endometriosis (presence of fragments of endometrial tissue in atypical locations for this tissue) or endometrial hyperplasia (excessive growth of the endometrium) in the past;
- increased risk of blood clots (see below "Blood clots in the veins (venous thromboembolic disease)");
- increased risk of estrogen-dependent tumors, e.g., breast cancer in the mother, sister, or grandmother;
- high blood pressure;
- liver disease (e.g., liver tumor);
- diabetes;
- gallstones;
- migraine or (severe) headaches;
- systemic immune system disease affecting many organs (systemic lupus erythematosus);
- epilepsy;
- asthma;
- otosclerosis (ear disease leading to progressive hearing loss);
- mastopathy;
- high triglyceride levels in the blood;
- fluid retention due to impaired heart or kidney function;
- a condition in which the thyroid gland does not produce enough thyroid hormones (hypothyroidism) and the patient is taking thyroid hormone replacement therapy;
- a hereditary or acquired condition that causes recurring episodes of swelling (hereditary and acquired angioedema) or a history of sudden swelling of the hands, face, feet, lips, eyes, tongue, throat (airway obstruction) or gastrointestinal tract.
If any of the following conditions occur during the use of HRT, you should stop using Systen 50 and contact your doctor immediately:
- if any of the conditions listed in the "When Not to Use Systen 50" section occur;
- if the skin or whites of the eyes turn yellow (jaundice), which may be a sign of liver function disorders;
- if you experience a significant increase in blood pressure (symptoms may include headache, fatigue, and dizziness);
- if you experience a migraine-type headache for the first time;
- if you become pregnant;
- if you experience facial swelling, tongue and/or throat swelling, and/or difficulty swallowing or hives, along with difficulty breathing, which may indicate angioedema;
- if you experience symptoms of a blood clot, such as:
- painful swelling and redness of the legs,
- sudden chest pain,
- difficulty breathing.
Note:Systen 50 is not a contraceptive. If it has been less than 12 months since your last menstrual period or you are under 50 years old, you may need to use an additional method of contraception. You should talk to your doctor.
Hormone Replacement Therapy and Cancer
Endometrial Hyperplasia and Endometrial Cancer
In women with an intact uterus, the risk of endometrial hyperplasia and endometrial cancer increases when estrogens are used alone for extended periods. It has been observed that in women using estrogen-only therapy, the risk of endometrial cancer is 2 to 12 times higher compared to women not using these medicinal products, depending on the duration of treatment and estrogen dose (see section 4). After stopping treatment, the increased risk may persist for at least 10 years.
Cyclic administration of additional progestogen for 12 days per month during a 28-day treatment cycle or continuous administration of a combination of estrogen and progestogen in women with an intact uterus prevents the increased risk associated with estrogen-only HRT.
In the first few months of treatment, there may be bleeding or spotting. If such symptoms occur after some time from the start of therapy or persist after its completion, you should tell your doctor, who will perform additional tests.
In women with an intact uterus who cannot tolerate progestogens, unopposed estrogen therapy may be considered, with a recommendation for long-term observation, including endometrial biopsies, performed annually or more frequently in case of bleeding or spotting.
Unopposed progestogen estrogen stimulation may lead to pre-cancerous or cancerous changes in persistent endometriosis foci. Therefore, in women who have had a hysterectomy due to endometriosis, especially if it is known that endometriosis foci have persisted, progestogen should be added to estrogen replacement therapy.
The safety of using transdermal systems > 50 μg per day, with added progestogen, has not been investigated.
Unexpected Bleeding
During the use of Systen 50, the patient will experience monthly bleeding (so-called withdrawal bleeding). If unexpected bleeding or spotting occurs in the patient, which:
- persists for longer than the first 6 months;
- occurs after using Systen 50 for more than 6 months;
- persists after stopping the use of Systen 50; you should contact your doctor as soon as possible.
Breast Cancer
Data confirm that taking hormone replacement therapy (HRT) in the form of a combination of estrogen and progestogen or estrogen alone increases the risk of breast cancer. The additional risk depends on how long the patient uses HRT. This additional risk becomes apparent after 3 years of HRT use. After stopping HRT, the additional risk will decrease over time, but the risk may persist for 10 years or longer if HRT lasted more than 5 years.
Comparison
In women aged 50-54 who do not use HRT, breast cancer is diagnosed in approximately 13 to 17 out of 1000 women over a 5-year period.
In women aged 50 who start a 5-year estrogen-only HRT, the number of cases will be 16-17 out of 1000 patients (i.e., 0 to 3 additional cases).
In women aged 50 who start a 5-year estrogen-progestogen HRT, the number of cases will be 21 out of 1000 patients (i.e., 4 to 8 additional cases).
In women aged 50-59 who do not use HRT, breast cancer is diagnosed in approximately 27 out of 1000 women over a 10-year period.
In women aged 50 who start a 10-year estrogen-only HRT, the number of cases will be 34 out of 1000 patients (i.e., 7 additional cases).
In women aged 50 who start a 10-year estrogen-progestogen HRT, the number of cases will be 48 out of 1000 patients (i.e., 21 additional cases).
You Should Regularly Examine Your Breasts. You Should Contact Your Doctor If You Notice Any of the Following Changes:
- wrinkling of the skin;
- changes in the nipple;
- any noticeable or palpable lumps.
In addition, it is recommended to participate in offered breast cancer screening programs.
It is essential to inform the nurse or medical staff performing the X-ray examination about the use of hormone replacement therapy, as this medicine may increase breast density, which can affect the mammography result. Not all lumps can be detected during a mammography examination in areas with increased breast density.
Ovarian Cancer
Ovarian cancer occurs less frequently than breast cancer. In some epidemiological studies, during long-term (at least 5 to 10 years) use of hormone replacement therapy, including only estrogens, an increased risk of ovarian cancer has been found in women who have had a hysterectomy.
Some studies, including the WHI study, suggest that long-term use of combined HRT may cause similar or slightly lower risk.
Hormone Replacement Therapy and Its Effect on the Heart and Circulation
Blood Clots in the Veins (Venous Thromboembolic Disease)
The use of HRT is associated with an increased risk of venous thromboembolic disease, i.e., deep vein thrombosis or pulmonary embolism.
Studies have shown a 2-3-fold increase in the risk of venous thromboembolic disease in women taking hormone replacement therapy compared to women not using it.
The occurrence of this complication is more likely in the first year of HRT use than later.
Blood clots can be life-threatening and, if they move to the lungs, can cause chest pain, shortness of breath, loss of consciousness, and even death.
The risk of blood clots in the veins is higher if the patient is older and if the following factors occur. You should inform your doctor if:
- you are unable to walk for a long time due to severe injuries or surgical procedures (see section 3. If surgery is planned);
- you are obese (body mass index - BMI >30 kg/m);
- you have thromboembolic disorders that require long-term use of anticoagulant medications;
and
- if anyone in your close family has had blood clots in the legs, lungs, or other organs in the past;
- if you have systemic lupus erythematosus.
If you experience symptoms of a blood clot, see "If any of the following conditions occur during the use of HRT, you should stop using Systen 50 and contact your doctor immediately".
There is no consensus on the potential role of varicose veins in venous thromboembolic disease.
Women already using anticoagulant therapy should discuss the balance of benefits and risks of using HRT with their doctor.
Hormone Replacement Therapy and Coronary Heart Disease
There is no evidence that HRT prevents myocardial infarction.
In women over 60 years old who use estrogen-progestogen HRT, the risk of coronary heart disease is slightly higher than in women not using HRT.
In women who have had a hysterectomy and are taking estrogen-only HRT, there is no increased risk of coronary heart disease.
Hormone Replacement Therapy and Stroke
The risk of stroke is about 1.5 times higher in women using HRT compared to women not using it.
The number of additional cases of stroke caused by HRT will increase with age.
Comparison
In women aged 50-59 who do not use HRT, the estimated number of stroke cases over 5 years is 8 out of 1000 women. In women aged 50-59 who use HRT, the number of cases over 5 years will be 11 out of 1000 women (i.e., 3 additional cases).
Conditions Requiring Observation During Estrogen Therapy:
- liver disorders or mild liver failure;
- jaundice with bile stasis in the medical history;
- HRT does not improve cognitive function. There is evidence of an increased risk of probable dementia in women who start treatment with continuous, combined, or estrogen-only HRT at an age over 65.
In women with an intact uterus using unopposed estrogen therapy, an increased risk of endometrial hyperplasia and endometrial cancer has been found. Therefore, to reduce the risk of endometrial hyperplasia and cancer, it is recommended to administer estrogen in combination with progestogen in women with an intact uterus, as during the use of Systen 50.
Systen 50 should be stored in a place inaccessible to children and pets.
Children and Adolescents
Systen 50 should not be used in children.
Systen 50 and Other Medicines
Certain medicines may affect the efficacy of Systen 50, which can lead to irregular bleeding. These include:
- antiepileptic drugs (such as phenobarbital, phenytoin, and carbamazepine),
- antituberculosis drugs (such as rifampicin, rifabutin),
- medicines used to treat HIV infection (such as nevirapine, efavirenz, ritonavir, and nelfinavir),
- medicines used to treat hepatitis C (such as telaprevir)
- herbal products containing St. John's Wort (Hypericum perforatum).
Hormone replacement therapy with estrogens may affect the action of other medicines:
- antiepileptic drugs (lamotrigine), as it may increase the frequency of seizures.
- medicines used to treat hepatitis C (such as ombitasvir/paritaprevir/ritonavir and dasabuvir with ribavirin or without ribavirin, glecaprevir/pibrentasvir, or sofosbuvir/velpatasvir/voxilaprevir) may cause increased liver function test results in the blood (increased ALT enzyme activity) in women using combined hormonal contraceptives containing ethinyl estradiol. Systen 50 contains estradiol instead of ethinyl estradiol. It is not known whether increased ALT enzyme activity may occur when using Systen 50 with such a treatment regimen.
You should tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take, including those available without a prescription, herbal medicines, and natural products. Your doctor will provide you with appropriate guidance.
Lab Tests
If you need to have a blood test, you should inform your doctor or laboratory staff that you are using Systen 50, as it may affect the results of some tests, such as glucose tolerance tests or thyroid function tests.
Pregnancy and Breastfeeding
Systen 50 is contraindicated during pregnancy. If you become pregnant while using Systen 50, you should stop using it immediately.
The use of Systen 50 is contraindicated during breastfeeding.
Driving and Using Machines
No studies have been conducted on the effect of Systen 50 on the ability to drive and use machines.
3. How to Use Systen 50
This medicine should always be used as directed by your doctor. If you are unsure, consult your doctor.
HRT should only be continued for as long as the benefits of alleviating severe symptoms outweigh the risks of using HRT.
Your doctor should prescribe the lowest possible dose for use for the shortest possible time to alleviate symptoms. If you think the dose of the medicine is too high or too low, you should consult your doctor.
Dosage
Your doctor will adjust the dose of Systen 50 according to its efficacy in a given woman.
Systen 50 should be used twice a week.
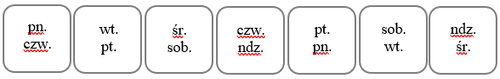
Patches should be changed every 3 or 4 days. The medicine should be used in a 3-week cycle, followed by a 7-day break. During this period, bleeding from the genital tract may occur. For example, if the application of the first patch occurs on Monday, it should be replaced on Thursday, and then on the following Monday. When determining these days, you can use the following table based on the first day of patch application:
| Day of application of the first patch | Day of patch replacement | Day of next patch replacement | ||
| Monday | → | Thursday | → | Monday |
| Tuesday | → | Friday | → | Tuesday |
| Wednesday | → | Saturday | → | Wednesday |
| Thursday | → | Sunday | → | Thursday |
| Friday | → | Monday | → | Friday |
| Saturday | → | Tuesday | → | Saturday |
| Sunday | → | Wednesday | → | Sunday |
To make it easier to remember the days of patch replacement, you can mark their configuration in the designated place on the packaging:
Continuous treatment with Systen 50 may be indicated in women who have had a hysterectomy or in whom severe estrogen deficiency symptoms occur during the break in treatment.
Treatment should be started with one patch of Systen 50.
The dose of the medicine should be adjusted based on the severity of estrogen overdose symptoms (adverse reactions) or lack of therapeutic effect.
In maintenance treatment, the doctor will recommend the lowest effective dose.
In women who have had a hysterectomy, the dose should not exceed 100 μg of estradiol per day.
In women with an intact uterus, the dose should not exceed 50 μg of estradiol per day.
In women who have had a hysterectomy, except for those with previously diagnosed endometriosis, it is not recommended to add progestogen to estrogen replacement therapy.
In the treatment of postmenopausal symptoms, the doctor will recommend the lowest effective dose.
If the patch is partially or completely detached, a new patch should be applied as soon as possible. However, the same day of patch replacement should be maintained.
In all women with an intact uterus, it is recommended to use progestogen administered in the following way:
- for 12 to 14 consecutive days per month during continuous use of Systen 50
or
- for the last 12 to 14 days (i.e., starting on the 8th or 10th day of the cycle) of a 21-day cycle of using Systen 50.
In both cases, bleeding from the genital tract usually occurs within 12 days of the first progestogen dose.
In patients with previously diagnosed endometrial hyperplasia (including women who have had a hysterectomy), during treatment with Systen 50, progestogen should be added periodically. Bleeding will occur after adding progestogen.
In women with an intact uterus treated with combined HRT, using one patch for longer than 4 days or staying without a patch attached for any period (missing a dose) increases the likelihood of intermenstrual bleeding or spotting.
Using Systen 50 in Patients with Renal or Hepatic Impairment
There are insufficient data on dosing in patients with severe hepatic or renal impairment.
Estrogens may cause fluid retention in the body, so patients with heart or kidney failure should be closely monitored during the use of Systen 50.
Using Systen 50 in Elderly Women
There are insufficient data to support the use of Systen 50 in women over 65 years old.
Method of Administration
| The patch should be applied to clean, dry, healthy, and intact skin on the trunk below the waist. Cream, body lotion, or powder may interact with the adhesive layer of the patch and should not be used at the site of application. Systen 50 should not be applied to the skin of the breasts or their area. The site where the patch is applied should be changed. To apply the patch to the same site, a minimum of one week should be allowed to pass. | 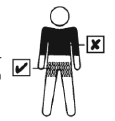 |
| Systen 50 should be used immediately after opening the sachet. | 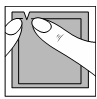 |
| After opening the sachet containing the patch, one part of the protective foil should be removed. | 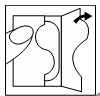 |
| Then, avoiding bending, the exposed part of the patch should be applied to the site where it will be attached, from its edge to the center. | 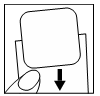 |
| The patient should remove the second part of the protective foil and press the patch onto the skin with their palm, again avoiding bending, and press the patch onto the skin for at least 10 seconds, warming it with the palm of their hand to body temperature, which is essential for optimal patch adhesion. When applying the patch, the patient should not directly touch the adhesive layer. | 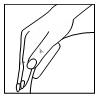 |
| It is not necessary to remove the patch during bathing or showering. However, it is recommended to remove the patch before using a sauna, and after the sauna, a new patch should be applied immediately. To remove the patch, you should grasp its edge and gently peel it off the skin. After use, the patch should be folded in half and thrown away in the trash (do not flush down the toilet). Any residual adhesive on the skin after removing the patch can be removed with soap and water or rubbed off with your fingers. | 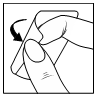 |
Using a Higher Than Recommended Dose of Systen 50
If you use a higher dose than recommended, you should immediately consult your doctor or pharmacist.
Symptoms of overdose may include: breast pain and tenderness, nausea, intermenstrual bleeding, abdominal cramps, and bloating. The mentioned symptoms are temporary and disappear without specific treatment after removing the patch.
Missing a Dose of Systen 50
If you forget to change the patch, you should apply the missed patch as soon as possible.
However, you should maintain the original schedule for changing the patch. Forgetting to use the medicine may increase the likelihood of intermenstrual bleeding or spotting.
If Surgery is Planned
If you are scheduled to have surgery, you should tell your surgeon that you are using Systen 50. It may be necessary to stop using Systen 50 4 to 6 weeks before surgery to minimize the risk of blood clots (see section 2. Blood clots in the veins (venous thromboembolic disease)). Before resuming the use of Systen 50, you should consult your doctor.
4. Possible Side Effects
Like all medicines, Systen 50 can cause side effects, although not everybody gets them.
In women using HRT, the following diseases occur more frequently compared to women not using HRT:
- breast cancer;
- endometrial hyperplasia or cancer;
- ovarian cancer;
- blood clots in the veins of the legs or lungs (venous thromboembolic disease);
- coronary heart disease;
- stroke;
- possibly dementia if HRT is started at an age over 65.
For more information, see section 2 "Important information before using Systen 50".
The following side effects have been observed in clinical trials and reported after the marketing of the transdermal system, Systen 50 patch:
Very common(may affect more than 1 in 10 people):
- itching at the application site, rash at the application site.
Common(may affect up to 1 in 10 people):
- low mood,
- migraine, dizziness, headache,
- abdominal pain, diarrhea, nausea,
- itching, rash,
- joint pain,
- breast pain, uterine bleeding,
- pain, redness at the application site, swelling at the application site, reaction at the application site,
- weight gain.
Uncommon(may affect up to 1 in 100 people):
- vaginal candidiasis,
- hypersensitivity (allergy),
- palpitations,
- bloating,
- muscle pain,
- breast enlargement, menstrual disorders,
- edema, generalized edema, peripheral edema.
Rare(may affect up to 1 in 1000 people):
- breast cancer,
- epilepsy,
- thrombosis,
- abdominal distension,
- gallstones.
Frequency not known(frequency cannot be estimated from the available data)
- endometrial cancer (cancer of the lining of the uterus),
- cerebrovascular incident,
- myocardial infarction,
- deep vein thrombosis,
- pulmonary embolism,
- angioedema.
The type of side effects, their frequency, and severity in women with an intact uterus treated with Systen 50 and progestogen differ depending on the type and dose of progestogen used with Systen 50.
Other side effects associated with oral estrogen-progestogen replacement therapy:
- dizziness;
- nausea, vomiting;
- leg or muscle pain, myasthenia;
- breast tenderness, uterine cramps, vaginal infection;
- uterine fibroids, ovarian cyst;
- increased liver enzyme activity - aminotransferases - in tests;
- gallbladder disease, jaundice;
- skin and subcutaneous tissue disorders: chloasma, erythema multiforme, erythema nodosum, purpura, acne, dry skin, alopecia;
- probable dementia in women over 65 years old;
- dry eye syndrome;
- change in tear composition.
Reporting Side Effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to Store Systen 50
Store at a temperature not exceeding 25°C.
The medicine should be stored in a place out of sight and reach of children.
This recommendation also applies to used transdermal systems, patches.
Do not use this medicine after the expiry date stated on the packaging.
The expiry date (EXP) refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the Pack and Other Information
What Systen 50 Contains
Each transdermal system, Systen 50 patch, contains 3.2 mg of estradiol (Estradiolum) in the form of estradiol hemihydrate as the active substance.
The other ingredients of the medicine are:
- Adhesive layer
- Absorbent-gum
- Polyester foil
What Systen 50 Looks Like and Contents of the Pack
6 or 8 transdermal systems, patches, placed in closed, foil sachets in a cardboard box.
The Systen 50 transdermal system, patch with an area of 16 cm² contains 3.2 mg of estradiol and releases 50 μg of estradiol over 24 hours.
Marketing Authorization Holder:
Theramex Ireland Limited
3rd Floor, Kilmore House,
Park Lane, Spencer Dock,
Dublin 1
D01 YE64
Ireland
Manufacturer:
Aesica Pharmaceuticals GmbH
Alfred-Nobel-Str. 10
40789 Monheim am Rhein
Germany
To obtain more detailed information about this medicine, you should contact the local representative of the marketing authorization holder Theramex, at phone number:
22 307 71 66.
Date of last revision of the leaflet:June 2025
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterAesica Pharmaceuticals GmbH LTS Lohmann Terapie-Systeme AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Sisten 50Dosage form: Gel, 0.5 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: Gel, 1 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: Gel, 1 mg/gActive substance: estradiolPrescription required
Alternatives to Sisten 50 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sisten 50 in Spain
Alternative to Sisten 50 in Ukraine
Online doctors for Sisten 50
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sisten 50 – subject to medical assessment and local rules.










